Arora, S.; Hoye, T.R. Org. Lett. 2021, 23, 3349–3353.
Abstract
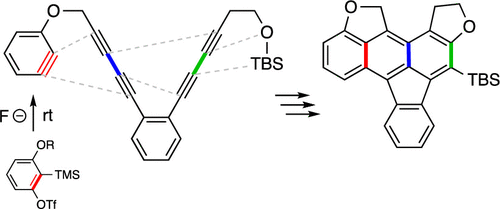
We report here elaboration of a cascade strategy for naphthyne formation using Kobayashi benzynes as diynophiles in a subsequent in situ hexadehydro-Diels–Alder reaction. Density functional theory computations suggest that the strained benzynes act as “super-diynophiles” in this transformation. The reaction requires only mildly basic and ambient temperature conditions and allows for rapid construction of various naphthalenic products. The trapping efficiencies of several arynophiles were determined using the benzyne to naphthyne cyclization as an internal clock reaction.