Sneddon, S.D.; Hoye, T.R. Org. Lett. 2021, 23, 3432–3436.
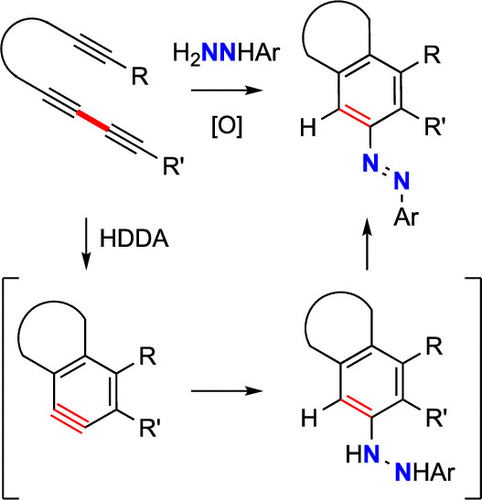
Arylhydrazines (ArNαHNβH2) are ambident nucleophiles. We describe here their reactivity with benzynes generated in situ by thermal cyclization of several multiynes. Products arising from attack of both the alpha- and beta-nitrogen atoms are observed. These competitive modes of reaction were explored by DFT calculations. Substituent effects on the site-selectivity for several substituted phenylhydrazines were explored. Interestingly, the hydrazo products from beta-attack (ArNHNHAr′) can be oxidized, sometimes in situ by oxygen alone, to give structurally complex, unsymmetrical azoarenes (ArN═NAr′). Toluenesulfonohydrazide and benzohydrazide analogues were each demonstrated to undergo similar transformations, including oxidation to the corresponding benzyne-trapped azo compounds.