Shen, H.; Xiao, X.; Haj, M. K.; Willoughby, P. H.; Hoye, T. R. J. Am. Chem. Soc. 2018, 140, 15616–15620.
Abstract
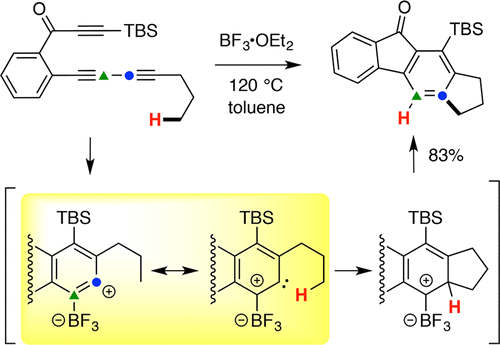
Boron trifluoride is observed to promote a variety of C–H insertion reactions of benzynes bearing pendant alkyl groups. Computations and various mechanistic studies indicate that BF3 engages the strained π-bond to confer carbene-like character on the adjacent, non-coordinated benzyne carbon. This represents an unprecedented catalytic role for a non-transition metal like BF3.