Brown, S. G.; Jansma, M. J.; Hoye, T. R. J. Nat. Prod. 2012, 75, 1326–1331.
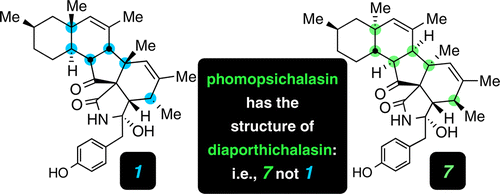
Phomopsichalasin was isolated and assigned structure 1 over 15 years ago. Analysis of its proton NMR data led us to hypothesize that not all aspects of the relative configuration of this structure were correct. We have used both empirical and computational methods to propose an alternative structure. Diaporthichalasin was reported several years ago, and its structure was assigned as 7, a diastereomer of structure 1, and confirmed by a single-crystal X-ray study. We have shown that diaporthichalasin and phomopsichalasin are identical; that is, both have structure 7. Additional aspects of NMR interpretation that provide guidance for avoiding some of the pitfalls that can lead to incorrect structure assignments are discussed. These recommendations/reminders include (i) the use of complementary solvents for acquiring NMR data that break accidental chemical shift degeneracy, (ii) the importance of assigning coupling constants as extensively as possible, and (iii) exercising caution when interpreting correlations in 2D spectra where overlapping resonances are involved.