Lopes, D. T.; Hoye, T. R.; Alvarenga, E. S. Magn. Reson. Chem. 2021, 59, 43–51.
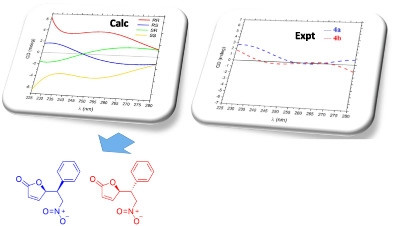
In the present work, we describe the preparation of two diastereomers from the enantioselective Michael addition of furan‐2(5H )‐one to (E )‐(2‐nitrovinyl)benzene catalyzed by a dinuclear Zn‐complex. The relative configurations of the diastereomeric products were assigned by comparing nuclear magnetic resonance (NMR) experimental chemical shift data with those computed by density functional theory (DFT) methods. Corrected mean absolute error (CMAE) and CP3 analyses were used to compare the data sets. The absolute configuration of each diastereomer was initially assigned by analysis of electronic circular dichroism (ECD) data, which was consistent with that of the known X‐ray crystallographic structure of the product of a related reaction, namely, (R )‐5‐((R )‐1‐(4‐chlorophenyl)‐2‐nitroethyl)furan‐2(5H )‐one.