Niu, D.; Hoye, T. R. Org. Lett. 2012, 14, 828–831.
Highlighted in the following editorial: Terpenoid- and shikimate-derived natural product total synthesis: A personal analysis and commentary on the importance of the papers that appear in this virtual issue. Hale, K. J. Org. Lett. 2013, 15, 3181–3198.
Abstract
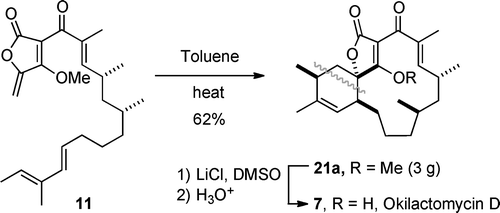
The spirotetronate okilactomycin D (7) has been efficiently synthesized by a route featuring a substrate-controlled, diastereoselective (8:1) intramolecular Diels–Alder (IMDA) reaction of 11. The assigned absolute configuration of (−)-7 was confirmed.