Chinta, B. S.; Lee, D.; Hoye, T. R. Org. Lett. 2021, 23, 2189–2193.
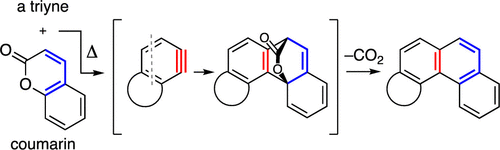
Although the parent 2-pyrone is known to react with simple o-benzynes to produce naphthalene derivatives, there appear to be no examples of the successful reaction of coumarin, a benzo-annulated 2-pyrone analogue, with an aryne. We report such a process here using benzynes generated by the hexadehydro-Diels–Alder reaction to produce phenanthrene derivatives (i.e., benzo-annulated naphthalenes). Density functional theory computations were used to help understand the difference in reactivity between 2-pyrone and the slower trapping agent, coumarin. Finally, the reaction of o-benzyne itself [from o-(trimethylsilyl)phenyl triflate and CsF] with coumarin was shown to be viable, although slow.