Wang, Q.; Hoye, T.R. Org. Lett. 2021, 23, 5448–5451.
Abstract
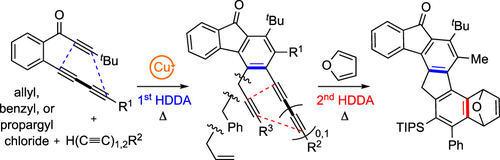
We report here a three-component, Cu(I)-catalyzed hexadehydro-Diels–Alder (HDDA) benzyne 1,2-difunctionalization reaction. This protocol allowed the introduction of two different carbon-based substituents onto the in situ-generated benzyne. These substituents were terminal monoynes or diynes partnered with propargylic, benzylic, or allylic chlorides. An example of a sequential HDDA reaction is demonstrated using the product of a 1,3-diyne and a propargylic halide, itself a newly created HDDA precursor.