Wang, T.; Hoye, T. R. Nature Chem. 2015, 7, 641–645.
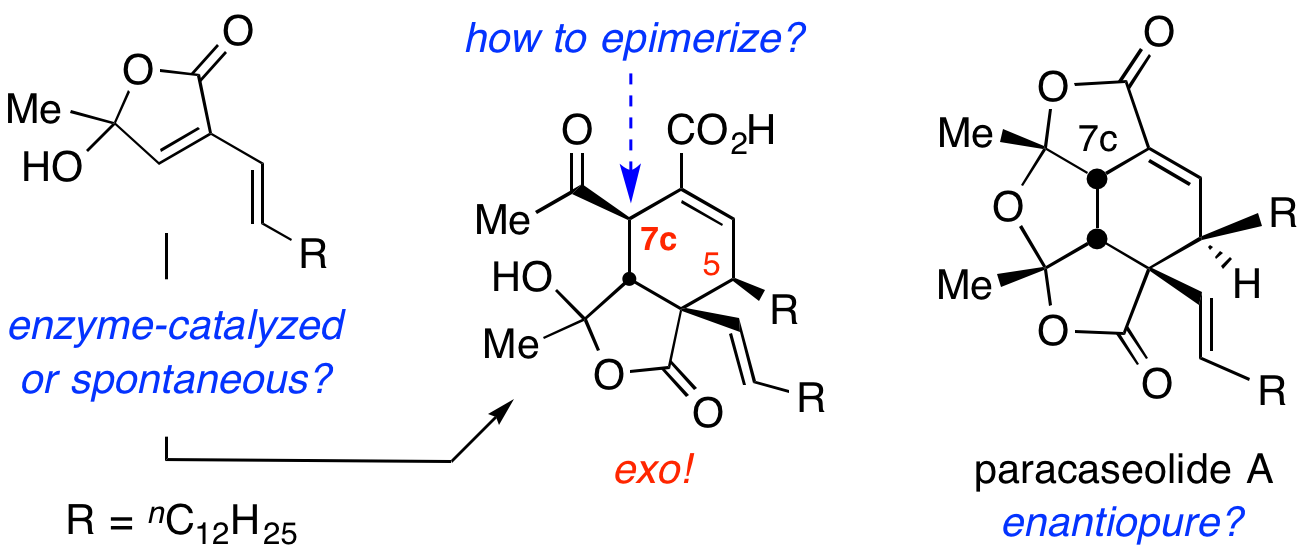
The natural product paracaseolide A is a tetracyclic dilactone containing six adjacent stereocentres. Its skeleton occupies a unique structural space among the >200,000 characterized secondary metabolites. Six different research groups have reported a chemical synthesis of this compound, five of which used a thermal, net Diels–Alder [4+2] cycloaddition and dehydration at 110 °C to access the target by dimerization of a simple butenolide precursor. Here, we report that this dimerization proceeds under much milder conditions and with a different stereochemical outcome than previously recognized. This can be rationalized by invoking a bis-pericyclic transition state. Furthermore, we find that spontaneous epimerization, necessary to correct the configuration at one key stereocentre, is viable and that natural paracaseolide A is racemic. Together, these facts point to the absence of enzymatic catalysis (that is, Diels–Alderase activity) in the cycloaddition and strongly suggest that a non-enzyme-mediated dimerization is the actual event by which paracaseolide A is produced in nature.