Xiao, X.; Hoye, T. R. Nature Chem. 2018, 10, 838–844.
Highlighted in Synfacts 2018, 14, 1035. (DOI: 10.1055/s-0037-1610940)
and in Chem 2018, 4, 2272–2274. (DOI: 10.1016/j.chempr.2018.09.021)
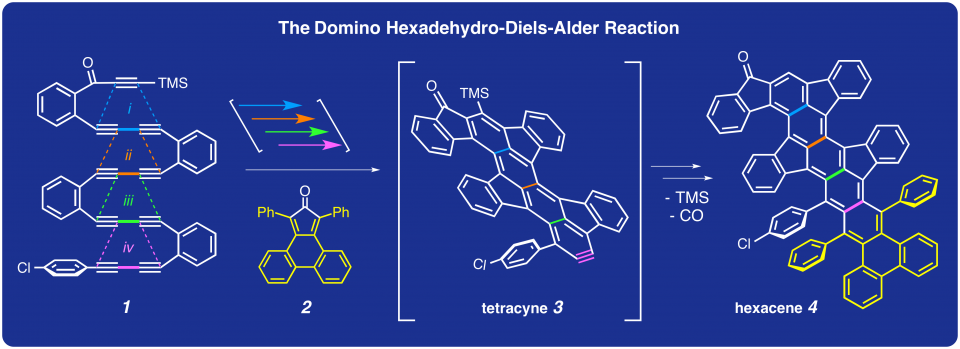
Polyacenes are organic compounds that have multiple, fused, aromatic rings. These highly conjugated molecules often have interesting photonic and/or electronic properties that afford them the potential for application in a host of organoelectronic devices such as sensors, light-emitting diodes, photovoltaic devices and field-effect transistors. Here, we show the development and use of the domino hexadehydro-Diels–Alder reaction to synthesize structurally diverse polyacenes from acyclic polyyne precursors. The key event in these transformations is the successive reaction of multiple 1,3-butadiyne units with a series of in-situ-generated, diynophilic arynes. The polyyne substrates were designed to allow for rapid engagement of each progressively larger aryne following the initiating (and ratelimiting) production of the first reactive intermediate—the benzyne. We show that aryne-trapping reactions are broad in scope and that these cascade or domino processes can be quite efficient.