Hoye, T. R.; Baire, B.; Niu, D.; Willoughby, P. H.; Woods, B. P. Nature 2012, 490, 208–212.
Highlighted in Synfacts 2013, 9, 153. (DOI: 10.1005/s-0033-1318087)
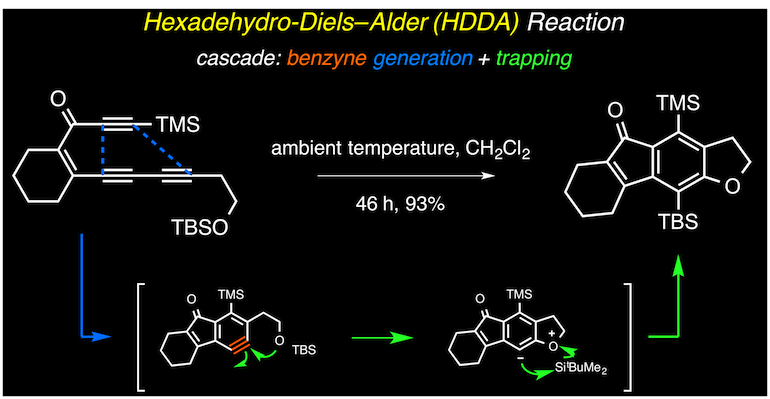
Arynes (aromatic systems containing, formally, a carbon–carbon triple bond) are among the most versatile of all reactive intermediates in organic chemistry. They can be ‘trapped’ to give products that are used as pharmaceuticals, agrochemicals, dyes, polymers and other fine chemicals. Here we explore a strategy that unites the de novo generation of benzynes—through a hexadehydro-Diels–Alder reaction—with their in situ elaboration into structurally complex benzenoid products. In the hexadehydro-Diels–Alder reaction, a 1,3-diyne is engaged in a [4+2] cycloisomerization with a ‘diynophile’ to produce the highly reactive benzyne intermediate. The reaction conditions for this simple, thermal transformation are notable for being free of metals and reagents. The subsequent and highly efficient trapping reactions increase the power of the overall process. Finally, we provide examples of how this de novo benzyne generation approach allows new modes of intrinsic reactivity to be revealed.