Li, K.; Brant, C.; Bussy, U.; Pinnamaneni, H.; Patel, H.; Hoye, T. R.; Li, W. Molecules 2015, 20, 5215–5222.
Abstract
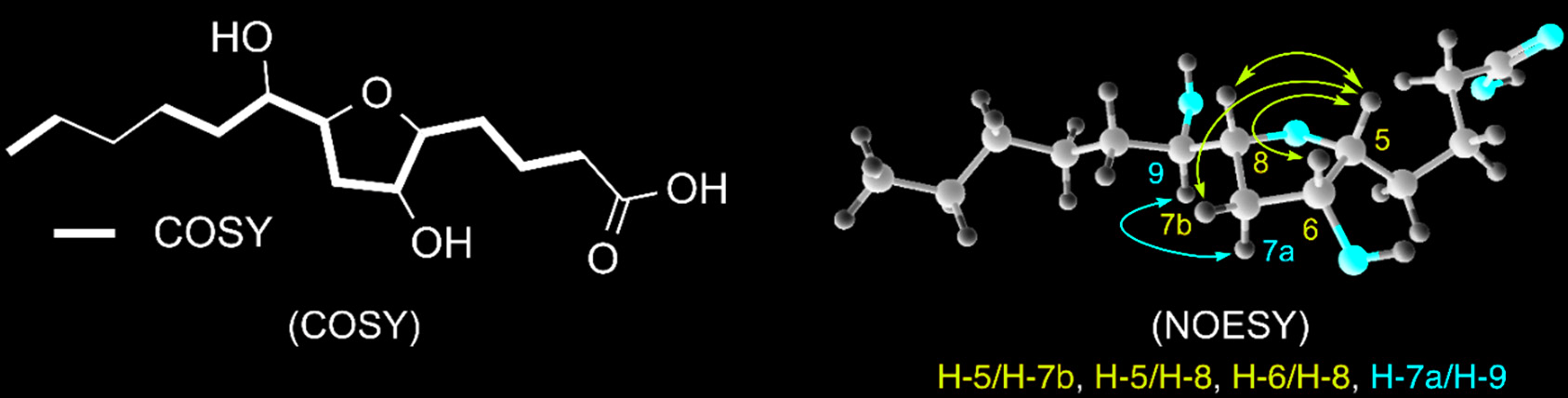
An enantiomeric pair of new fatty acid-derived hydroxylated tetrahydrofurans, here named iso-petromyroxols, were isolated from sea lamprey larvae-conditioned water. The relative configuration of iso-petromyroxol was elucidated with 1D and 2D NMR spectroscopic analyses. The ratio of enantiomers (er) in the natural sample was measured by chiral-HPLC-MS/MS to be ca. 3:1 of (–)- to (+)-antipodes.