Ross, S. P.; Hoye, T. R. Org. Lett. 2018, 20, 100–103.
Abstract
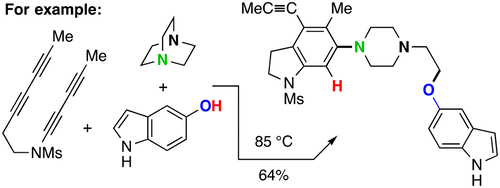
A broadly general, three-component reaction strategy for the construction of compounds containing multiple heterocycles is described. Thermal benzyne formation (by the hexadehydro-Diels–Alder (HDDA) reaction) in the presence of tertiary cyclic amines and a protic nucleophile (HNu) gives, via ring-opening of intermediate ammonium ion/Nu– ion pairs, heterocyclic products. Many reactions are efficient even when the stoichiometric loading of the three reactants approaches unity. Use of HOSO2CF3 as the HNu gives ammonium triflate intermediates, which can then be ring opened by an even wider variety of nucleophiles.