Xiao, X.; Hoye, T. R. J. Am. Chem. Soc. 2019, 141, 9813–9818.
Abstract
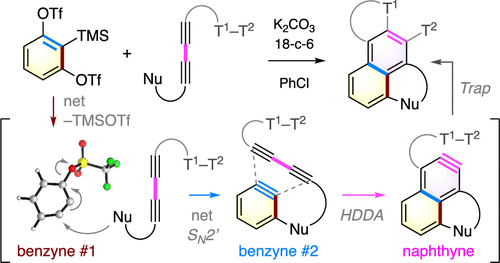
Here we disclose a cascade strategy for naphthyne formation that capitalizes on the traditional benzyne generation (i.e., from an ortho-silyl aryl triflate) and the thermal hexadehydro-Diels–Alder (HDDA) reaction. In this transformation, three distinct aryne species work in tandem, two of which can be formally considered as a 1,2-benzidyne, and each undergoes a different type of trapping event. Many examples were explored by varying the naphthyne capture chemistry as well as the 1,2-benzdiyne equivalent. This strategy enables rapid construction of various naphthalene products and has potential for the synthesis of extended polycyclic arenes.