Xu, S.; Wang, Y.; Hoye, T. R. Macromolecules 2020, 53, 4952–4959.
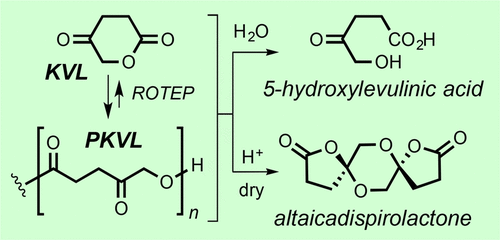
We report here the synthesis of poly(4-ketovalerolactone) (PKVL) via ring-opening transesterification polymerization (ROTEP) of the monomer 4-ketovalerolactone (KVL, two steps from levulinic acid). The polymerization of KVL proceeds to high equilibrium monomer conversion (up to 96% in the melt) to give the semicrystalline polyketoester PKVL. PKVL displays glass transition temperatures of 7 °C and two melting temperatures at 132 and 148 °C. This polyester can be chemically recycled through hydrolytic degradation. Under aqueous neutral or acidic conditions, the dominating pathway for polyester hydrolysis is through backbiting from the chain end. Under basic conditions, midchain cleavage, accelerated by the ketone carbonyl group in the backbone, promotes the hydrolysis of nearby backbone ester bonds. The final hydrolysis product is 5-hydroxylevulinic acid, the ring-opened hydrolysis product of KVL. PKVL was also observed to degrade under the action of a Brønsted acid to a bis-spirocyclic dilactone natural product altaicadispirolactone, which is a dimer of KVL. This constitutes a rare example of a one-step synthesis of a secondary metabolite of nontrivial structure in which a polymer was the starting material and the sole source of matter. Analogous ROTEP of the isomeric four-membered lactone 4-acetyl-β-propiolactone (APL) was also explored, although this chemistry was not as well-behaved as the KVL to PKVL polymerization.