Ball-Jones, N. R.; Fahnhorst, G. W.; Hoye, T. R. ACS Macro Lett. 2016, 5, 1128–1131.
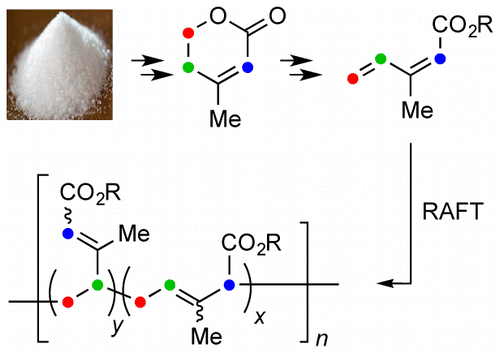
A short and efficient synthesis of a series of isoprenecarboxylic acid esters and their corresponding polymers is presented. The base-catalyzed eliminative ring opening of anhydromevalonolactone (3) provides isoprenecarboxylic acid (6-H), which was further transformed to the isoprenecarboxylic acid esters. Reversible addition–fragmentation chain-transfer (RAFT) polymerization was used to synthesize high molecular weight (>100 kg mol–1) poly(isoprenecarboxylates) with dispersities (Đ) of ca. 1.5. The glass transition temperatures (Tg) and entanglement molecular weights (Me) of the poly(isoprenecarboxylates) were determined and showed similar trends to the Tg and Me values for analogous poly(acrylate esters). These new glucose-derived materials could provide a sustainable alternative to poly(acrylates).