Lee, D.; Ross, S.P.; Xiao, X.; Hoye, T.R. Chem. 2021, 7, 2527–2537.
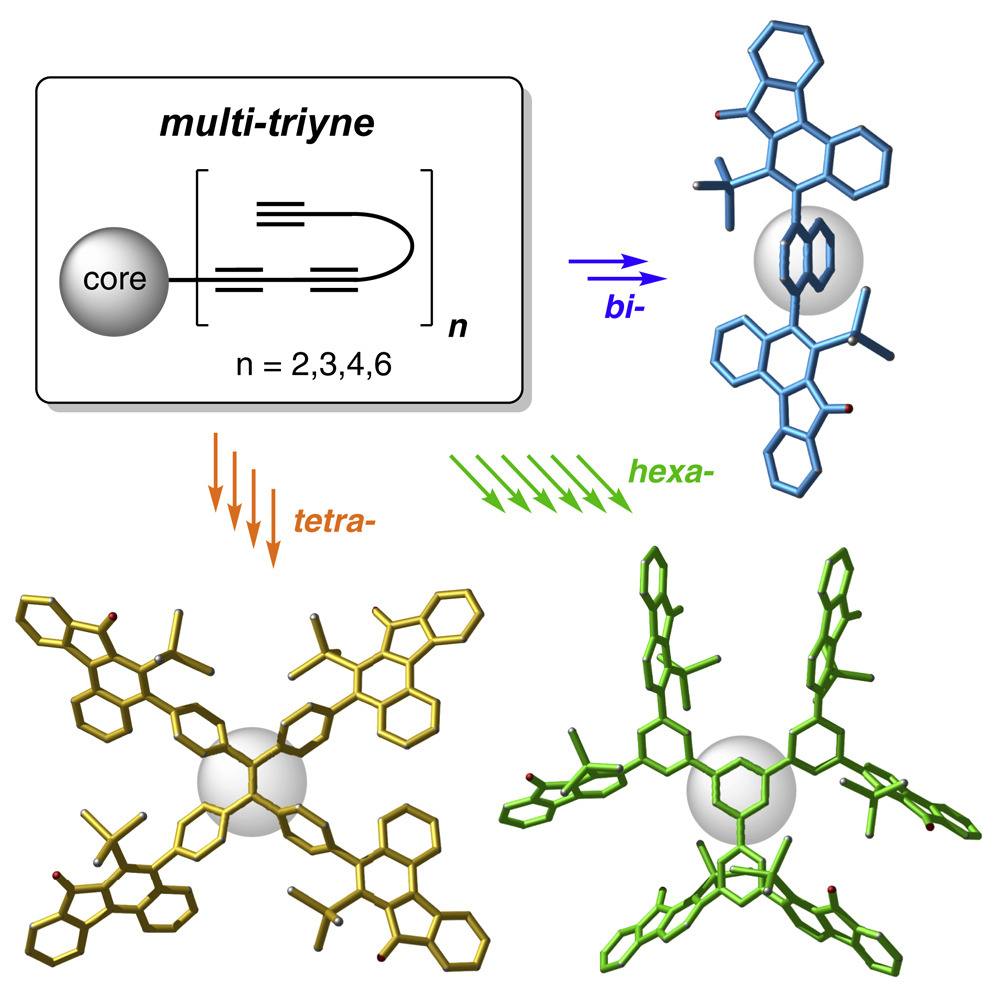
Polycyclic, highly fused, and, perforce, highly conjugated aromatic organic compounds have been of interest to chemists since the discovery of naphthalene in 1821. In modern decades these have attracted ever-growing attention because of their architectures, properties, and wide-ranging practical applications. Given the unabated interest in such molecules, the development of new methods and strategies for the practical synthesis of PACs having new structural motifs is important. Here, we describe one-pot, purely thermal cyclizations of substrates containing sets of independent triynes, each arrayed upon a common core structure. This produces topologically unique products through sequential generation/trapping of a series of benzyne intermediates. More specifically, these all conform to processes that can be considered as radial-hexadehydro-Diels-Alder (HDDA) reactions. The late-stage and de novo creation of multiple arenes in these multibenzyne processes constitutes a fundamentally new synthetic strategy for constructing novel molecular topologies.