Chen, J.; Palani, V.; Hoye, T. R. J. Am. Chem. Soc. 2016, 138, 4318–4321.
Abstract
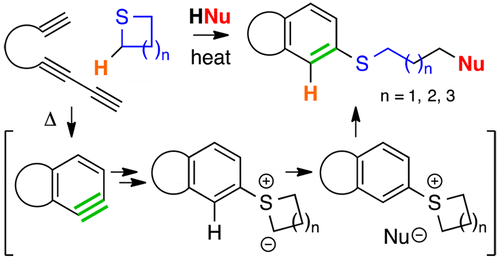
We report here reactions of alkyl sulfides with benzynes thermally generated by the hexadehydro-Diels–Alder (HDDA) cycloisomerization. The initially produced 1,3-betaine (o-sulfonium/aryl carbanion) undergoes intramolecular proton transfer to generate a more stable S-aryl sulfur ylide. This can react in various manners, including engaging weak acids (HA) in the reaction medium. This can produce transient ion pairs ArSR2+A– that proceed to the products ArSR + RA. When cyclic sulfides are used, A– opens the ring and is incorporated into the product, an outcome that constitutes a versatile, three-component coupling process.