Palani, V.; Chen, J.; Hoye, T. R. Org. Lett. 2016, 18, 6312–6315.
Abstract
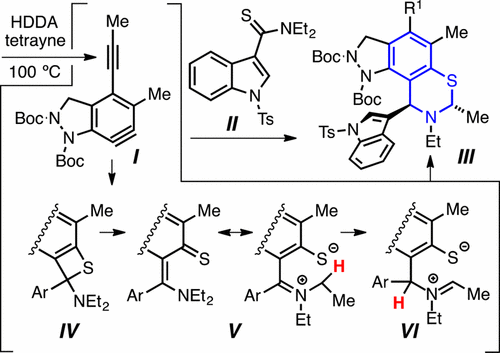
Reaction of thioamides (e.g., II) with benzynes generated by the hexadehydro-Diels–Alder (HDDA) cycloisomerization (e.g., I) produces dihydrobenzothiazines (e.g., III). It is postulated that the reaction proceeds via benzothietene (cf. IV) and o-thiolatoaryliminium (cf. V) intermediates and that the latter undergoes intramolecular 1,3-hydrogen atom migration to produce the penultimate isomeric iminium zwitterions VI.