Kraemer, N.; Naredla, R.R.; Hoye, T.R. Org. Lett. 2022, 24, 2327–2331.
Abstract
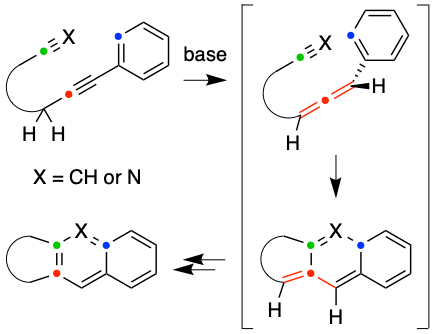
We have explored the net-[4 + 2]-cycloadditions of a variety of aryl (or alkenyl) alkynes. Tautomerization via base-catalyzed alkyne-to-allene isomerization produces a transient allene, which undergoes stepwise cyclization with not only a pendant alkyne but also a nitrile. The operative mechanisms for these reactions were studied by density functional theory and compared with the slower thermal cyclization of the precursor alkyne.