Wang, Y.; Zheng, L.; Hoye, T. R. Org. Lett. 2018, 20, 7145–7148.
Abstract
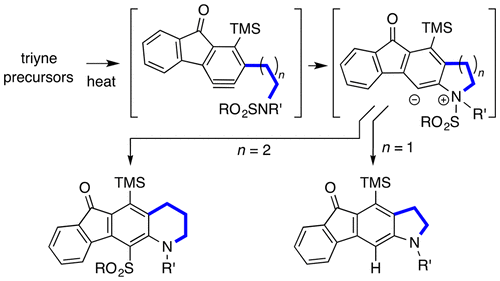
Reactions of tethered, tertiary sulfonamides with thermally generated benzynes are reported. Typically, the N–S bonds in the substrates cleave, and saturated heterocycles [tetrahydroquinolines (n = 2) and indolines (n = 1)] are formed. The process is accompanied by either sulfonyl transfer or desulfonylation from a zwitterionic intermediate, with the favored pathway being largely dependent upon the size (5- vs 6-membered) of the N-containing ring in the zwitterion.