Ritts, C.B.; Hoye, T.R. J. Am. Chem. Soc. 2021, 143, 13501–13506.
Highlighted in JACS Spotlights 2021, 143, 16875–16876. (DOI: 10.1021/jacs.1c10754)
and in Synfacts 2021, 17, 1213. (DOI: 10.1055/s-0040-1720748)
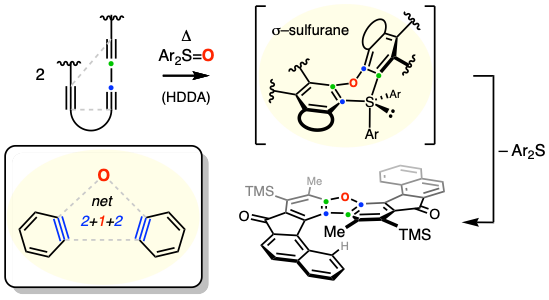
Here we disclose a sulfurane-mediated method for the formation of dimeric dibenzofuran helicenes via the reaction between diaryl sulfoxides and hexadehydro-Diels–Alder (HDDA) derived benzynes. A variety of S-shaped and U-shaped helicenes were formed under thermal conditions. Both experimental and DFT studies support a sulfur(IV)-based coupling (aka ligand coupling) mechanism involving tetracarbo-ligated S(IV) intermediates undergoing reductive elimination to afford the helicene products. This process involves the de novo generation of five new rings in a single operation and constitutes a new method for the construction of topologically interesting, polycyclic aromatic compounds.