Baire, B.; Niu, D.; Willoughby, P. H.; Woods, B. P.; Hoye, T. R. Nat. Protoc. 2013, 8, 501–508.
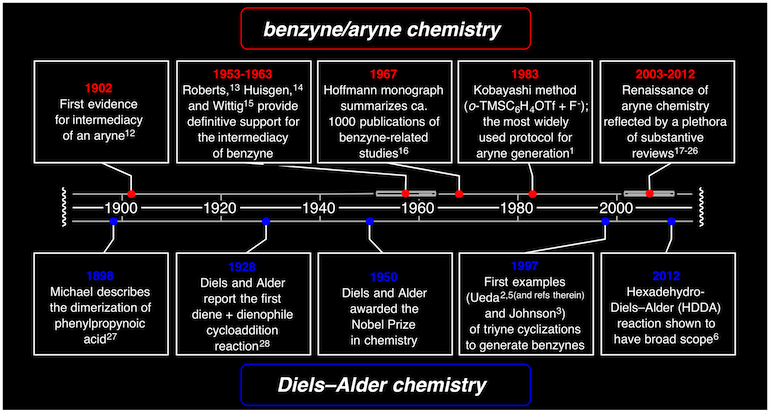
The hexadehydro-Diels-Alder (HDDA) cascade enables the synthesis of complex benzenoid products with various substitution patterns through aryne intermediates. The first stage of this cascade involves the generation of a highly reactive ortho-benzyne intermediate by a net [4+2] cycloisomerization of a triyne substrate. The benzyne can be rapidly 'trapped' either intramolecularly or intermolecularly with myriad nucleophilic or π-bond-donating reactants. As a representative example of a general procedure for synthesizing highly substituted benzenoids, this protocol describes the synthesis of a typical triyne substrate and its use as the reactant in an HDDA cascade to form a phthalide. The synthetic procedure detailed herein (four chemical reactions) takes 16–20 h of active effort over a period of several days for the preparation of the triyne precursor and ∼2 h of active effort over a 3-d period for the generation and trapping of the benzyne and isolation of the phthalide product.