Gormong, E.A.; Reineke, T.M.; Hoye, T.R. ACS Macro Lett. 2021, 10, 1068–1072.
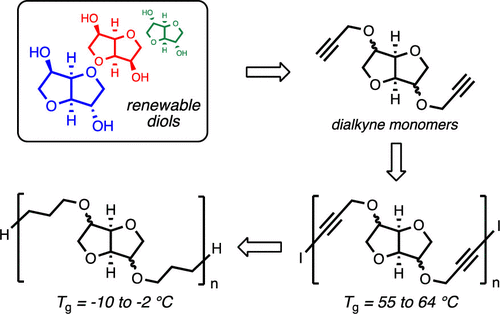
The incorporation of renewable feedstocks into polymer backbones is of great importance in modern polymer science. We report the synthesis of 1,3-diyne polymers derived from the bispropargyl ethers of isosorbide, isomannide, and isoidide. The dialkyne monomers can be polymerized through an adaptation of the Glaser–Hay coupling using a nickel(II) cocatalyst. These well-defined diyne polymers bear an iodoalkyne end group, afforded through an unanticipated reductive elimination pathway, and display glass transition temperatures (Tg) from 55 to 64 °C. Fully saturated, analogous polyethers can be prepared from the hydrogenation of the diyne polymers, and these show Tg values between −10 and −2 °C. Both the 1,3-diyne polymers and the saturated analogues display similar trends in their Tg values vis-à-vis the stereochemical features of the isohexide unit within the backbone. This polymerization provided access to two series of isohexide-based polyethers, the thermal properties of which are influenced by the nature of the 2,4-hexadiynyl and hexamethylene linkers as well as the relative configuration of the bicyclic subunit in the backbone. The reported method represents an important step toward accessing well-defined polyethers from renewable feedstocks using readily available catalysts and convenient ambient conditions.