Wang, T.; Oswood, C. J.; Hoye, T. R. Syn. Lett. 2017, 28, A-C.
Dedicated with very best wishes to Professor Victor Snieckus on the occasion of his 80th birthday.
Abstract
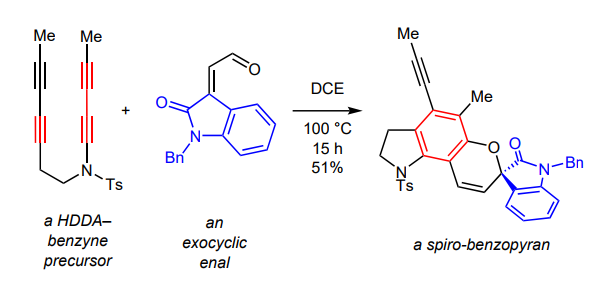
Exocyclic, conjugated enals react with benzynes generated by heating various triyne-containing substrates to produce spirocyclic benzopyran derivatives. These products are consistent with a mechanistic sequence that involves initial net [2+2] cycloaddition of the benzyne and aldehyde followed by 4π-electrocyclic ring opening and 6π ring closing.