Chinta, B.S.; Arora, S.; Hoye, T.R. Org. Lett. 2022, 24, 425–429.
Abstract
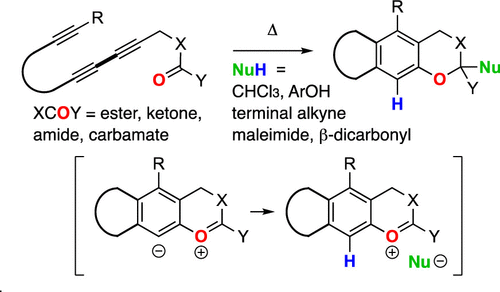
We describe here reactions in which a carbonyl oxygen atom initiates cascade reactions by nucleophilic attack on a covalently attached benzyne. The benzynes are produced by thermal cyclization of triynes via hexadehydro-Diels−Alder reaction. The initially produced oxocarbenium/aryl carbanionic zwitterion is protonated in situ by an external protic nucleophile (NuH) of appropriate acidity. The resulting ion pair (oxocarbenium+/Nu−) collapses through several different mechanistic manifolds, adding to the diversity of structural classes that can be generated.