Xiao, X.; Woods, B. P.; Xiu, W.; Hoye, T. R. Angew. Chem. Int. Ed. 2018, 57, 9901–9905.
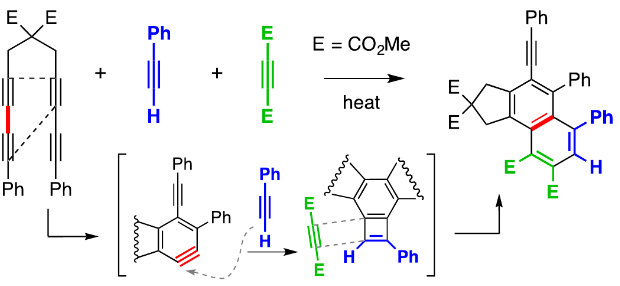
The reaction of an aryne with an alkyne to generate a benzocyclobutadiene (BCB) intermediate is rare. We report here examples of this reaction, revealed by Diels‐Alder trapping of the BCB by either pendant or external, electron‐deficient alkynes. Mechanistic delineation of the reaction course is supported by DFT calculations. A three‐component process joining a benzyne, first, with an electron‐rich and, then, an electron‐poor alkyne was uncovered. Reactions in which the BCB functions in a rarely observed role as a 4π diene component in Diels‐Alder reactions are reported. The results also shed new light on aspects of the hexadehydro‐Diels‐Alder reaction used to generate the benzynes.