Xu, F.; Hershey, K. W.; Holmes, R. J.; Hoye, T. R. J. Am. Chem. Soc. 2016, 138, 12739–12742.
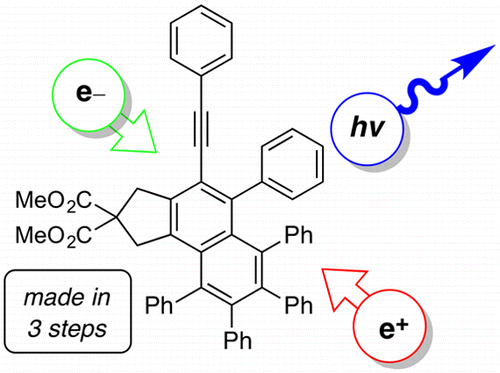
We describe here three alkynyl substituted naphthalenes that display promising luminescence characteristics. Each compound is easily and efficiently synthesized in three steps by capitalizing on the hexadehydro-Diels–Alder (HDDA) cycloisomerization reaction in which an intermediate benzyne is captured by tetraphenylcyclopentadienone, a classical trap for benzyne itself. These compounds luminesce in the deep blue when stimulated either optically (i.e., photoluminescence in both solution and solid films) or electrically [in a light-emitting diode (LED)]. The photophysical properties are relatively insensitive to the electronic nature of the substituents (H, OMe, CO2Me) that define these otherwise identical compounds. Overall, our observations suggest that the twisted nature of the five adjacent aryl groups serves to minimize the intermolecular interaction between core naphthalene units in different sample morphologies. These compounds represent promising leads for the identification of others of value as the emissive component of organic LEDs (OLEDs).