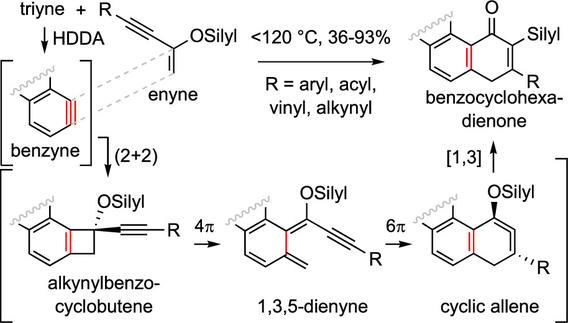
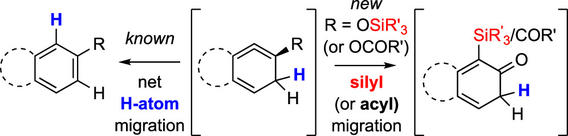
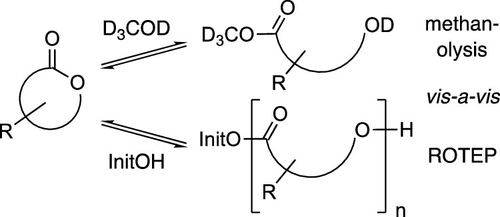
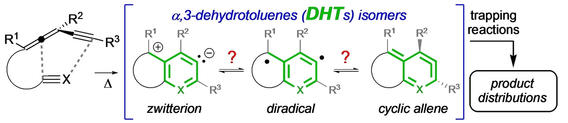
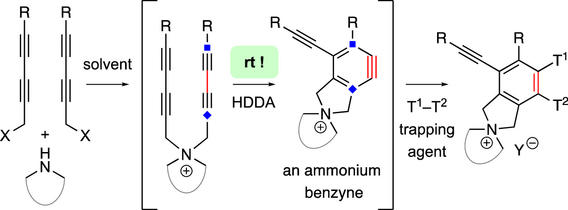
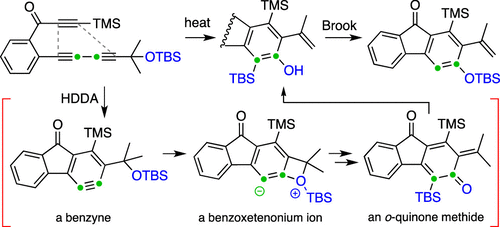
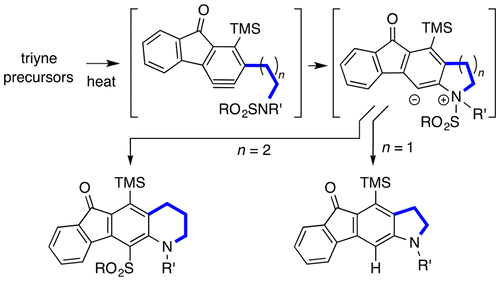
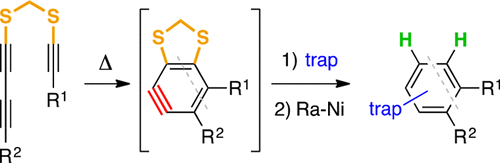
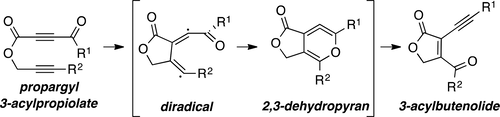
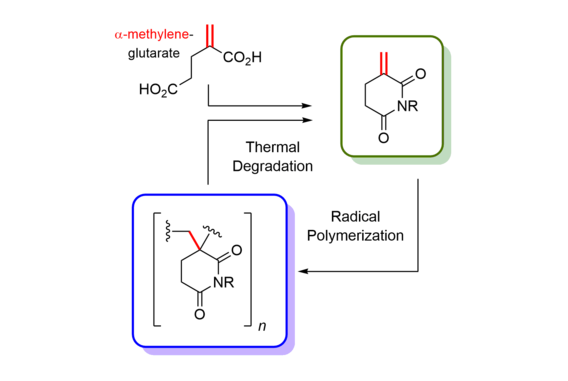
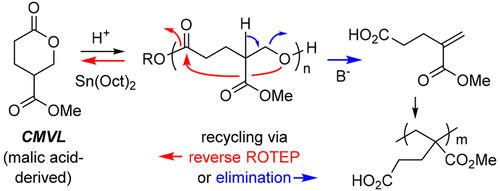
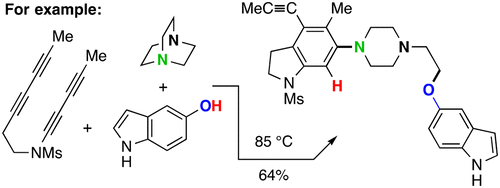
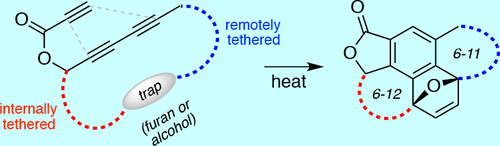
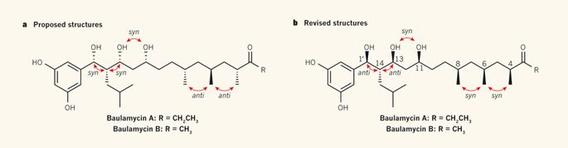
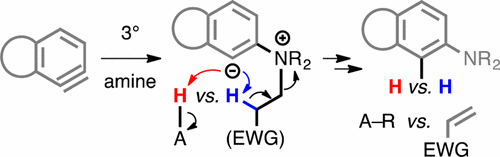
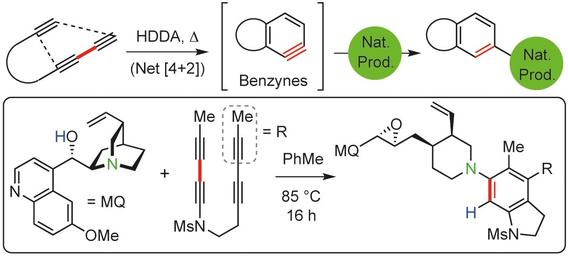
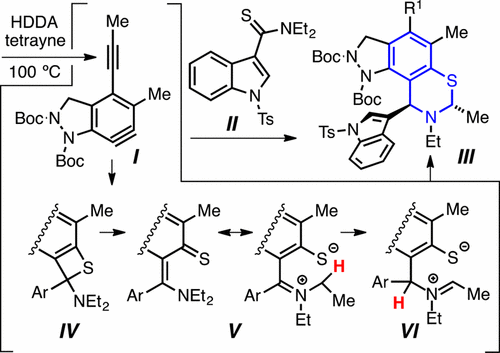
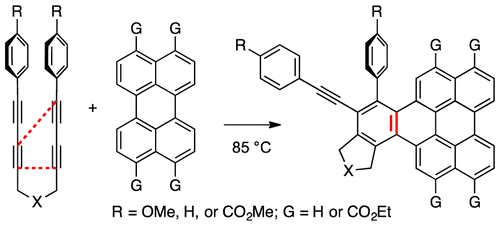
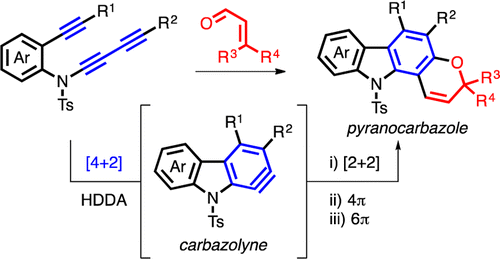
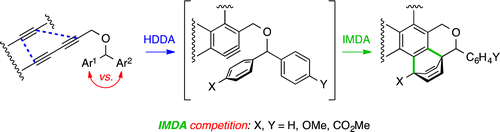
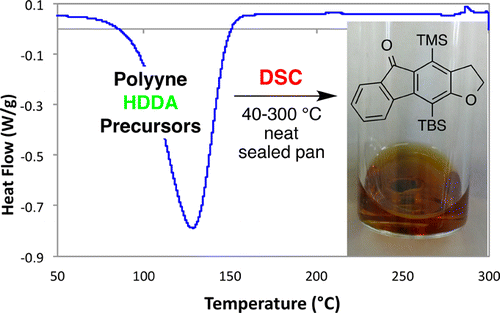
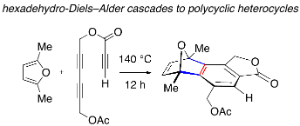
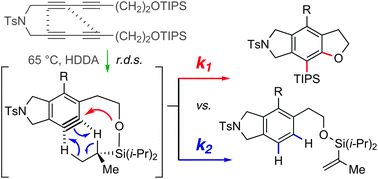
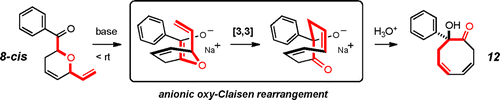
Cascade reactions of HDDA-benzynes with tethered cyclohexadienones: strain-driven events originating from ortho-annulated benzocyclobutenes
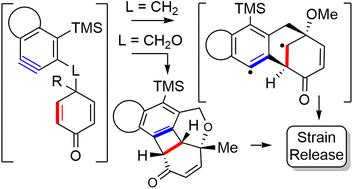
Chinta, B. S.; Sneddon, D. S.; Hoye, T. R. Chem. Sci. 2024, 15, 8181–8189.
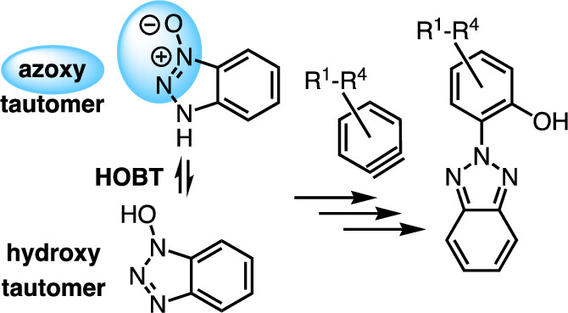
2:1 Adducts Arising from Reactions between Benzynes and 1,3,4-Oxadiazoles
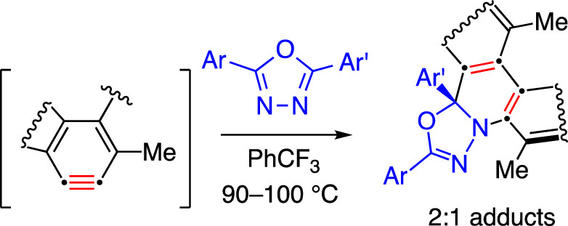
Guzman, A. L.; Kevorkian, P. V.; Hoye, T. R. Org. Lett. 2024, 26, 3834–3839.
Deciphering molecular structures: NMR spectroscopy and quantum mechanical insights of halogenated 4H‐Chromenediones
Martins, L. M. O. S.; Souto, F. T.; Hoye, T. R.; Alvarenga, E. S. Magn. Reson. Chem. 2024, 62, 583–598.
Phosphorane-Promoted C–C Coupling during Aryne Annulations
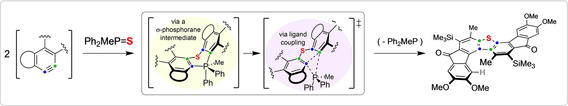
Kevorkian, P.V.; Sneddon, D.S.; Ritts, C.B.; Hoye, T.R. Angew. Chem. Int. Ed. 2024, e202318774.
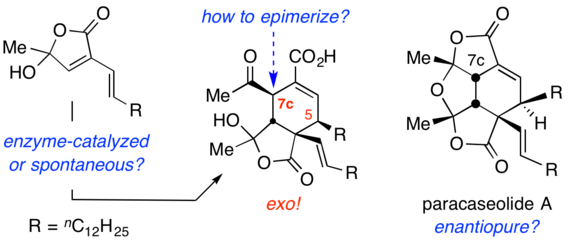
Novel Conversions of a Multifunctional, Bio-sourced Lactone Carboxylic Acid
Kaicharla, T.; Lee, S.; Wang, R.; Pehere, A. D.; Xu, S.; Hoye, T. R. Arkivoc 2024, 202312081.
Intramolecular Cyclization of Alkynylheteroaromatic Substrates Bearing a Tethered Cyano Group: A Strategy for Accessing Fused Pyridoheterocycles
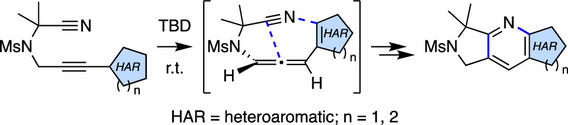
Kraemer, N.; Eason, E. M.; Hoye, T. R. J. Org. Chem. 2023, 88, 12716–12726.
Covalent Adduct Formation between β-Lactoglobulin and Flavor Compounds under Thermal Treatments That Mimic Food Pasteurization or Sterilization
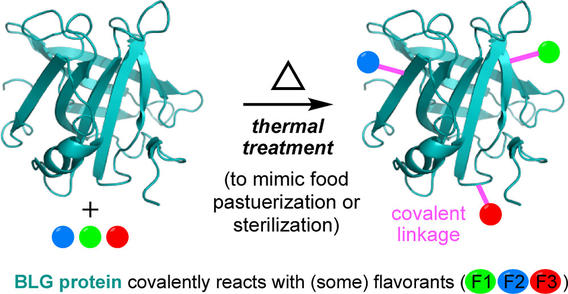
Yuan, J.; Anantharamkrishnan, V.; Hoye, T. R.; Reineccius, G. A. J. Agric. Food Chem. 2023, 71, 9481–9489.
Neighboring Group Effects on the Rates of Cleavage of Si–O–Si-Containing Compounds
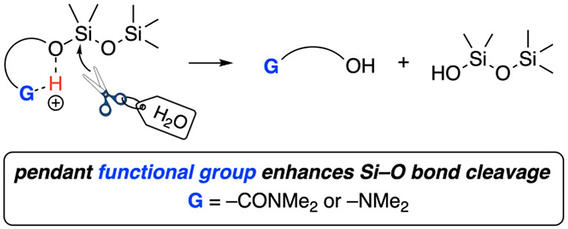
Gormong, E. A.; Sneddon, D. S.; Reineke, T. M.; Hoye, T. R. J. Org. Chem. 2023, 88, 1988–1995.
The Dual Therapeutic Potential of Ottelione A on Carbon Tetrachloride-induced Hepatic Toxicity in Mice
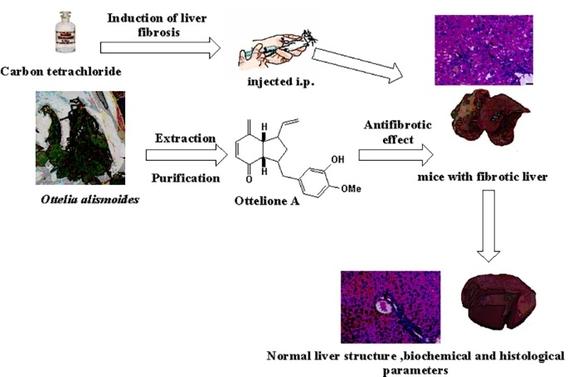
Zahran, R. F.; EL-sayed, L. M.; Hoye, T. R.; Ayyad, S-E. N. Appl. Biochem. Biotechnol. 2023, 195, 5966–5979.
Examples Showing the Utility of Doping Experiments in 1H NMR Analysis of Mixtures
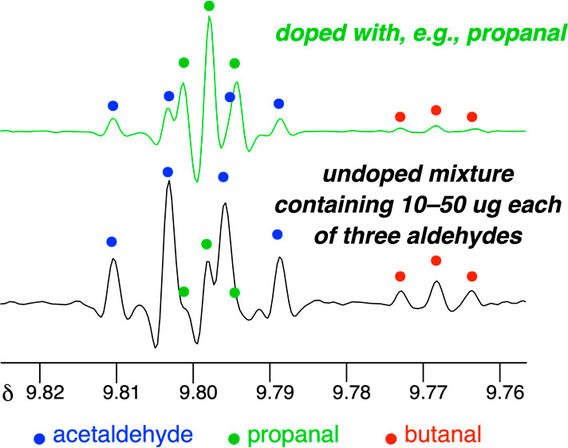
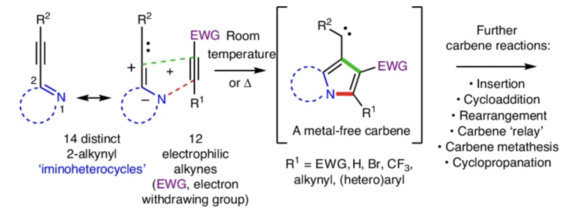
Kaicharla, T.; Chinta, B.S.; Hoye, T.R. J. Org. Chem. 2022, 87, 5660–5667.
In Situ Allene Formation via Alkyne Tautomerization to Promote [4 + 2]-Cycloadditions with a Pendant Alkyne or Nitrile
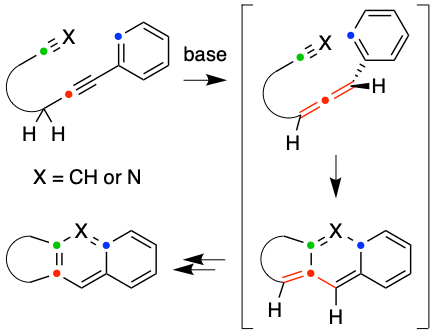
Kraemer, N.; Naredla, R.R.; Hoye, T.R. Org. Lett. 2022, 24, 2327–2331.
Defining the Macromolecules of Tomorrow through Synergistic Sustainable Polymer Research
Haque, F.M.; Ishibashi, J.S.A.; Lidston, C.A.L.; Shao, H.; Bates, F.S.; Chang, A.B.; Coates, G.W.; Cramer, C.J.; Dauenhauer, P.J.; Dichtel, W.R.; Ellison, C.J.; Gormong, E.A.; Hamachi, L.S.; Hoye, T.R.; Jin, M.; Kalow, J.A.; Kim, H.J.; Kumar, G.; LaSalle, C.J.; Liffland, S.; Lipinski, B.M.; Pang, Y.; Parveen, R.; Peng, X.; Popowski, Y.; Prebihalo, E.A.; Reddi, Y.; Reineke, T.M.; Sheppard, D.T.; Swartz, J.L.; Tolman, W.B.; Vlaisavljevich, B.; Wissinger, J.; Xu, S.; Hillmyer, M.A. Chem. Rev. 2022, 122, 6322–6373.
Hydrothermal catalysis of waste greases into green gasoline, jet, and diesel biofuels in continuous flow supercritical water
Fedie, R.L.; McNeff, C.V.; McNeff, C.V.; McNeff, L.C.; Greuel, P.G.; Yan, B.; Jenkins, J.A.; Brethorst, J.T.; Frost, G.B.; Hoye, T.R. Biofuels, Bioprod. Bioref. 2022, 16, 349–369.
Synthesis of a novel naphthalenone endoperoxide and structural elucidation by NMR spectroscopy and theoretical calculation
Martins, L.M.O.S.; Santos, J.O.; Hoye, T.R.; Alvarenga, E.S. Magn. Reson. Chem. 2022, 60, 139–147.
Sulfurane [S(IV)]-Mediated Fusion of Benzynes Leads to Helical Dibenzofurans
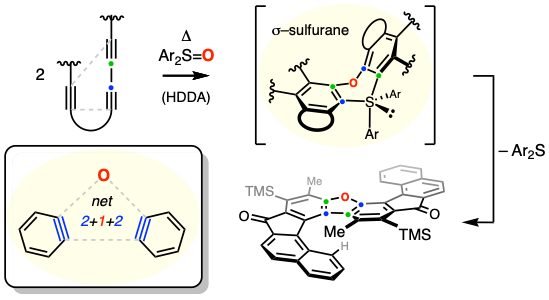
Ritts, C.B.; Hoye, T.R. J. Am. Chem. Soc. 2021, 143, 13501–13506.
Highlighted in JACS Spotlights 2021, 143, 16875–16876. (DOI: 10.1021/jacs.1c10754)
and in Synfacts 2021, 17, 1213. (DOI: 10.1055/s-0040-1720748)
Synthesis of Isohexide Diyne Polymers and Hydrogenation to Their Saturated Polyethers
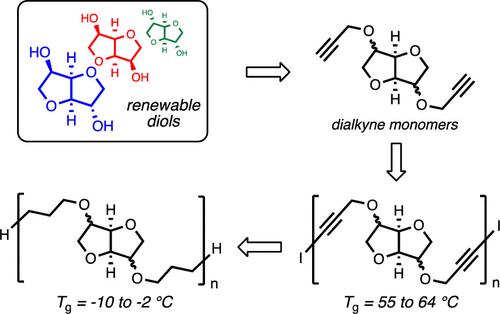
Gormong, E.A.; Reineke, T.M.; Hoye, T.R. ACS Macro Lett. 2021, 10, 1068–1072.
Hexadehydro Diels–Alder (HDDA) Route to Arynes and Related Chemistry
Voss, R.N.; Hoye, T.R. Hexadehydro Diels–Alder (HDDA) Route to Arynes and Related Chemistry. In Modern Aryne Chemistry; Biju, A.T.; Wiley-VCH: Weinheim, Germany, 2021; Chapter 10; pp 407–444.
β-Methyl-δ-valerolactone-containing thermoplastic poly(ester-amide)s: synthesis, mechanical properties, and degradation behavior
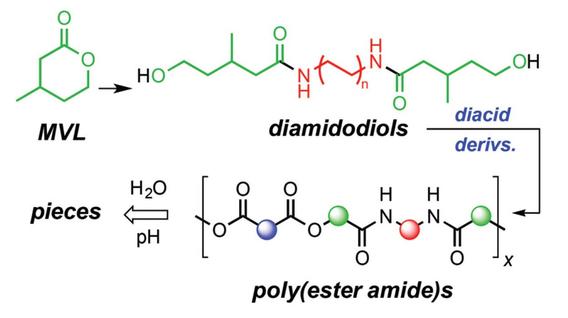
Guptill, D. M.; Chinta, B. S.; Kaicharla, T.; Xu, S.; Hoye, T. R. Polym. Chem. 2021, 12, 1310–1316.
Characterization of stereoisomeric 5‐(2‐nitro‐1‐phenylethyl)furan‐2(5H )‐ones by computation of 1H and 13C NMR chemical shifts and electronic circular dichroism spectra
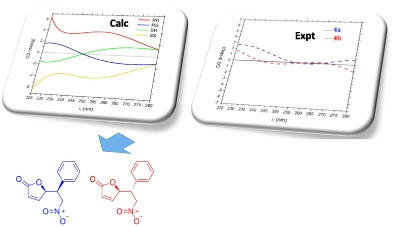
Lopes, D. T.; Hoye, T. R.; Alvarenga, E. S. Magn. Reson. Chem. 2021, 59, 43–51.
Poly(4-ketovalerolactone) from Levulinic Acid: Synthesis and Hydrolytic Degradation
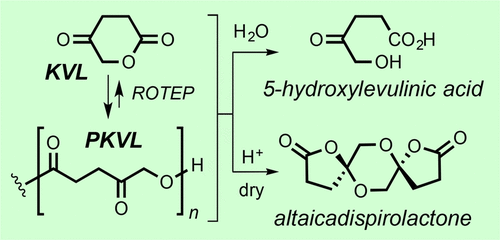
Xu, S.; Wang, Y.; Hoye, T. R. Macromolecules 2020, 53, 4952–4959.
Covalent Adduct Formation Between Flavor Compounds of Various Functional Group Classes and the Model Protein β-Lactoglobulin
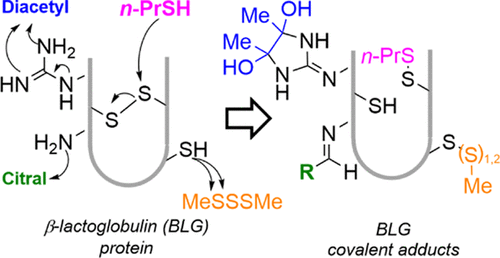
Anantharamkrishnan, V.; Hoye, T. R.; Reineccius, G. A. J. Agric. Food Chem. 2020, 68, 6395–6402.
4-Carboalkoxylated Polyvalerolactones from Malic Acid: Tough and Degradable Polyesters
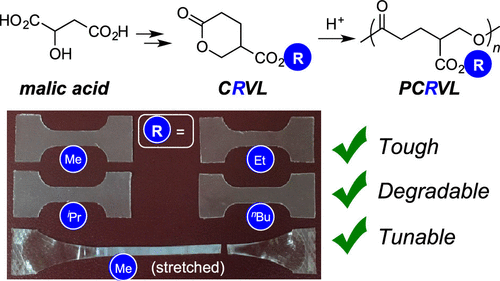
Fahnhorst, G. W.; De Hoe, G. X.; Hillmyer, M. A.; Hoye, T. R. Macromolecules 2020, 53, 3194–3201.
Reactions of HDDA Benzynes with C,N-Diarylimines (ArCH=NAr′)
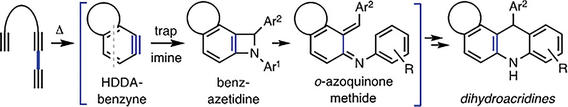
Arora, S.; Sneddon, D. S.; Hoye, T. R. Eur. J. Org. Chem. 2020, 2379–2383.
Addendum: A guide to small-molecule structure assignment through computation of (¹H and ¹³C) NMR chemical shifts
Willoughby, P.H.; Jansma, M.J.; Hoye, T.R. Nat. Protoc. 2020, 15, 2777.
The aza-hexadehydro-Diels-Alder (aza-HDDA) reaction
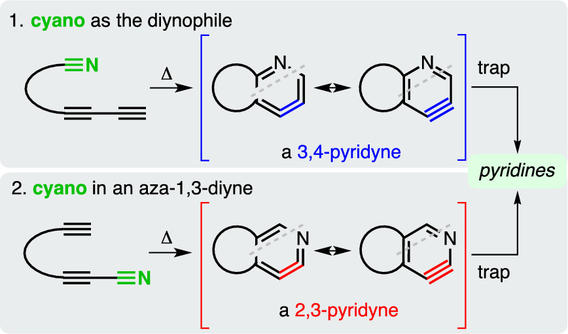
Thompson, S. K.; Hoye, T. R. J. Am. Chem. Soc. 2019, 141, 19575–19580.
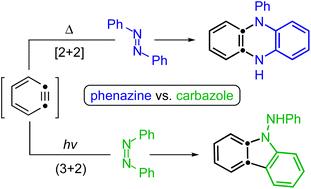
Reactions of thermally generated benzynes with six-membered N–heteroaromatics: Pathway and product diversity
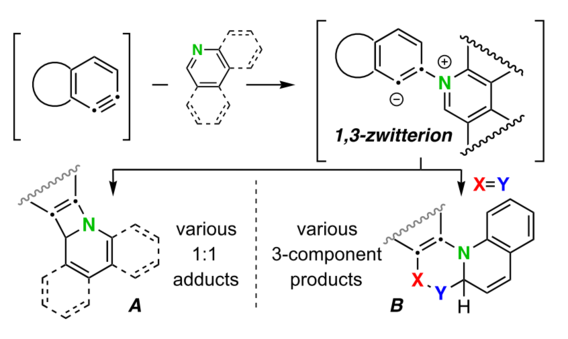
Arora, S.; Zhang, J.; Pogula, V.; Hoye, T. R. Chem. Sci. 2019, 10, 9069–9076.
Hydrolytically-degradable homo- and copolymers of a strained exocyclic hemiacetal ester
Neitzel, A. E.; Barreda, L.; Trotta, J. T.; Fahnhorst, G. W.; Haversang, T. J.; Hoye, T. R.; Fors, B. P.; Hillmyer, M. A. Polym. Chem. 2019, 10, 4573–4583.
Superabsorbent Poly(isoprenecarboxylate) Hydrogels from Glucose
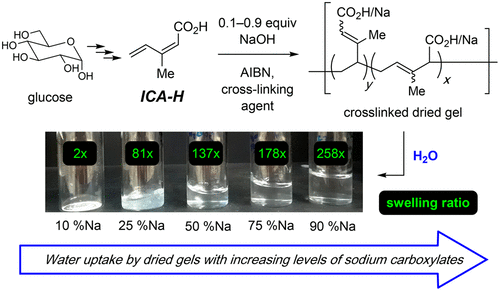
Fahnhorst, G. W.; Hoye, T. R. ACS Sustainable Chem. Eng. 2019, 7, 7491–7495.
Reactions of Diaziridines with Benzynes Give N‑Arylhydrazones
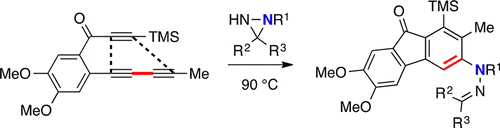
Arora, S.; Palani, V.; Hoye, T. R. Org. Lett. 2018, 20, 8082–8085.
This manuscript is dedicated in memory of Professor Harold W. Heine, formerly of Bucknell University, and in recognition of his considerable scientific and mentoring legacy, which has so positively impacted so many (including the outgoing Editor-in-Chief of Organic Letters).
Cu(I)-Mediated Bromoalkynylation and Hydroalkynylation Reactions of Unsymmetrical Benzynes: Complementary Modes of Addition
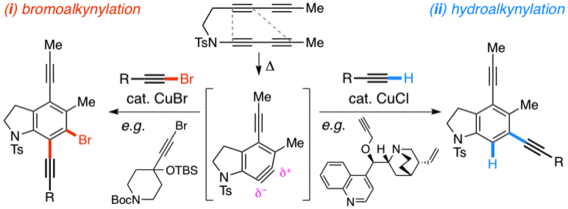
Xiao, X.; Wang, T.; Xu, F.; Hoye, T. R. Angew. Chem. Int. Ed. 2018, 57, 16564–16568.
BF3-Promoted, Carbene-like, C–H Insertion Reactions of Benzynes
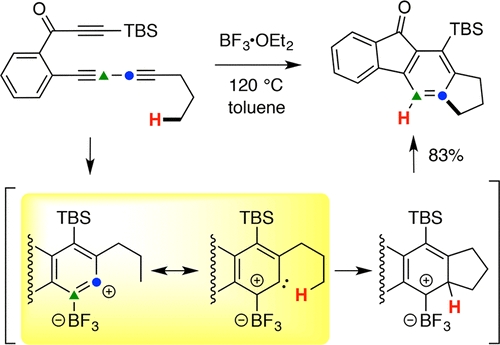
Shen, H.; Xiao, X.; Haj, M. K.; Willoughby, P. H.; Hoye, T. R. J. Am. Chem. Soc. 2018, 140, 15616–15620.
Isomerization of Linear to Hyperbranched Polymers: Two Isomeric Lactones Converge via Metastable Isostructural Polyesters to a Highly Branched Analogue
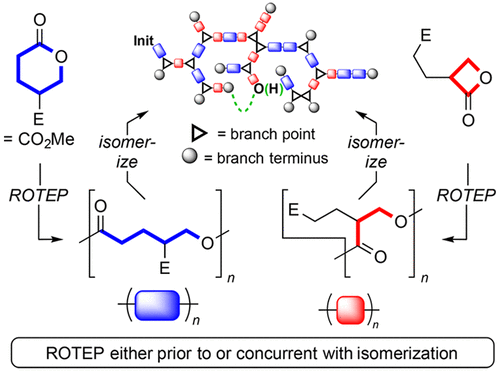
Fahnhorst, G. W.; Stasiw, D. E.; Tolman, W. B.; Hoye, T. R. ACS Macro Lett. 2018, 7, 1144–1148.
Atypical Mode of [3 + 2]-Cycloaddition: Pseudo-1,3-dipole Behavior in Reactions of Electron-Deficient Thioamides with Benzynes
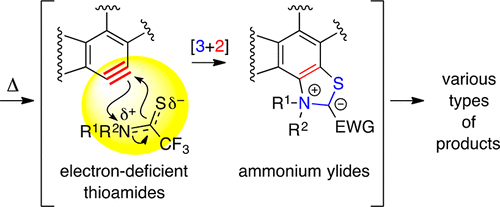
Zhang, J.; Page, A. C. S.; Palani, V.; Chen, J.; Hoye, T. R. Org. Lett. 2018, 20, 5550–5553.
Fatty-acid derivative acts as a sea lamprey migratory pheromone
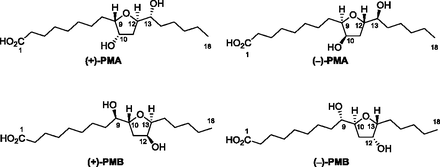
Li, K.; Brant, C. O.; Huertas, M.; Hessler, E. J.; Mezei, G.; Scott, A. M.; Hoye, T. R.; Li, W. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 8603–8608.
Unraveling substituent effects on the glass transition temperatures of biorenewable polyesters
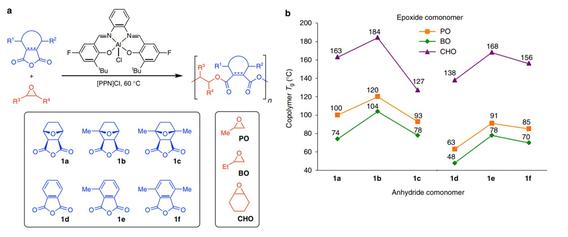
Yu, X.; Jia, J.; Xu, S.; Lao, K. U.; Sanford, M. J.; Ramakrishnan, R. K.; Nazarenko, S. I.; Hoye, T. R.; Coates, G. W.; DiStasio R. A. Jr. Nature Commun. 2018, 9, 2880.
The domino hexadehydro-Diels–Alder reaction transforms polyynes to benzynes to naphthynes to anthracynes to tetracynes (and beyond?)
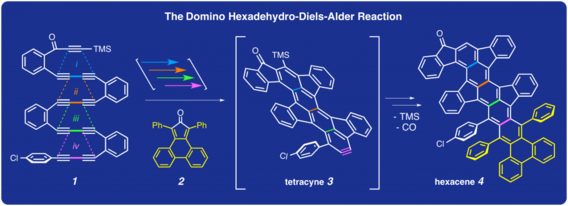
Xiao, X.; Hoye, T. R. Nature Chem. 2018, 10, 838–844.
Highlighted in Synfacts 2018, 14, 1035. (DOI: 10.1055/s-0037-1610940)
and in Chem 2018, 4, 2272–2274. (DOI: 10.1016/j.chempr.2018.09.021)
Benzocyclobutadienes: An unusual mode of access reveals unusual modes of reactivity
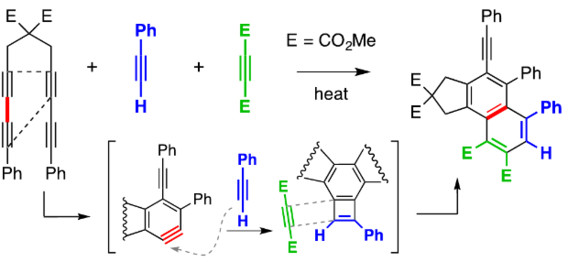
Xiao, X.; Woods, B. P.; Xiu, W.; Hoye, T. R. Angew. Chem. Int. Ed. 2018, 57, 9901–9905.
Engineering the Production of Dipicolinic Acid in E. coli
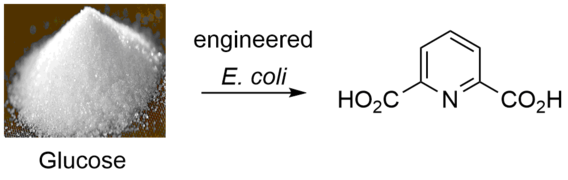
McClintock, M. K.; Fahnhorst, G. W.; Hoye, T. R.; Zhang, K. Metab. Eng. 2018, 48, 208–217.
Trapping of Hexadehydro-Diels–Alder Benzynes with Exocyclic, Conjugated Enals as a Route to Fused Spirocyclic Benzopyran Motifs
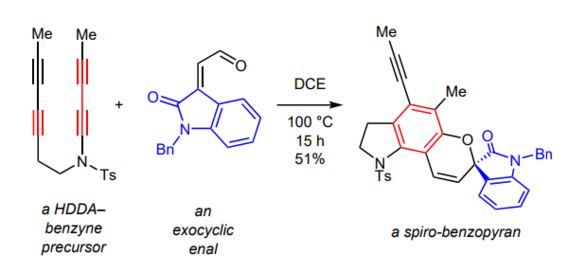
Wang, T.; Oswood, C. J.; Hoye, T. R. Syn. Lett. 2017, 28, A-C.
Dedicated with very best wishes to Professor Victor Snieckus on the occasion of his 80th birthday.
Bile Salt-like Dienones Having a Novel Skeleton or a Rare Substitution Pattern Function as Chemical Cues in Adult Sea Lamprey
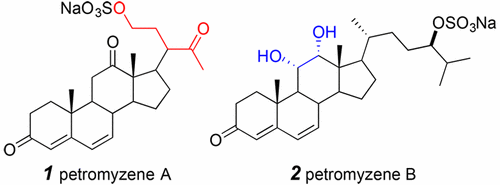
Li, K.; Scott, A.M.; Brant, C.O.; Fissette, S.D.; Riedy, J.J.; Hoye, T.R.; Li, W. Org. Lett. 2017, 19, 4444–4447.
The Photochemical Hexadehydro-Diels-Alder (hv-HDDA) Reaction
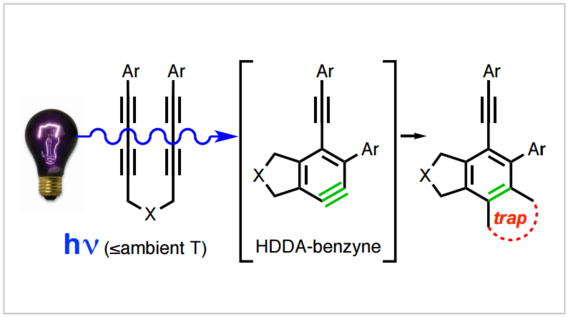
Xu, F.; Xiao, X.; Hoye, T. R. J. Am. Chem. Soc. 2017, 139, 8400–8403.
Highlighted in Synfacts 2017, 13, 809. (DOI: 10.1055/s-0036-1590716)
Antiparasitic Sesquiterpenes from the Cameroonian Spice Scleria striatinux and Preliminary In Vitro and In Silico DMPK Assessment
Nyongbela, K.D.; Ntie-Kang, F.; Hoye, T.R.; Efange, S.M.N. Nat. Prod. Bioprospect. 2017, 1–13.
Thermoplastic polyurethanes from b-methyl-d-valerolactone-derived amidodiol chain extenders
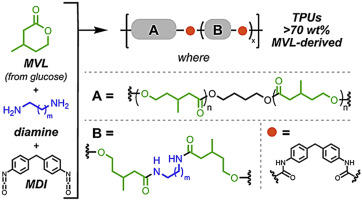
Guptill, D. M.; Brutman, J. P.; Hoye, T. R. Polymer 2017, 111, 252–257.
The phenol-ene reaction: Biaryl synthesis via trapping reactions between HDDA-generated benzynes and phenolics
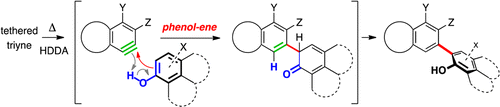
Zhang, J.; Niu, D.; Brinker, V. A.; Hoye, T. R. Org. Lett. 2016, 18, 5596–5599.
Poly(isoprenecarboxylates) from glucose via anhydromevalonolactone
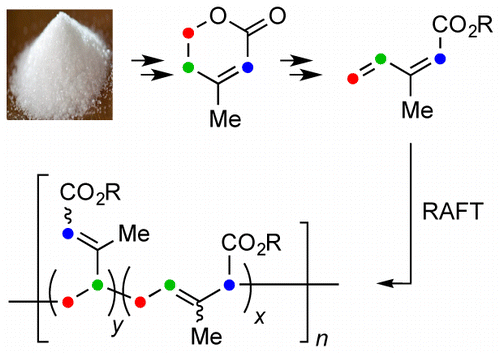
Ball-Jones, N. R.; Fahnhorst, G. W.; Hoye, T. R. ACS Macro Lett. 2016, 5, 1128–1131.
Blue-emitting arylalkynyl naphthalene derivatives via a hexadehydro-Diels-Alder (HDDA) cascade reaction
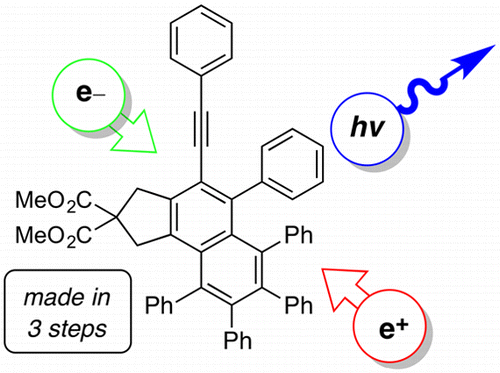
Xu, F.; Hershey, K. W.; Holmes, R. J.; Hoye, T. R. J. Am. Chem. Soc. 2016, 138, 12739–12742.
The hexadehydro-Diels–Alder (HDDA) cycloisomerization reaction proceeds by a stepwise mechanism
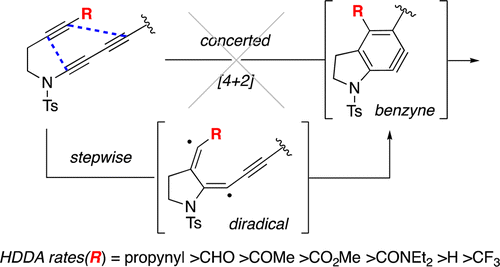
Wang, T.; Niu, D.; Hoye, T. R. J. Am. Chem. Soc. 2016, 138, 7832–7835.
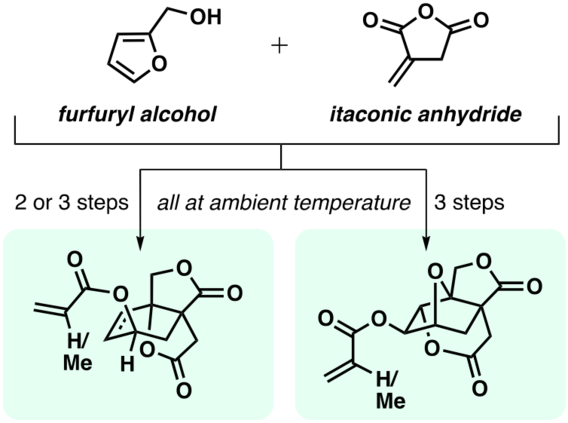
Diels-Alder reactions of furans with itaconic anhydride: Overcoming unfavorable thermodynamics
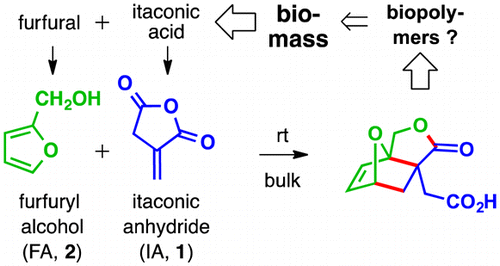
Pehere, A. D.; Xu, S.; Thompson, S. K.; Hillmyer, M. A.; Hoye, T. R. Org. Lett. 2016, 18, 2584–2587.
The pentadehydro-Diels–Alder reaction
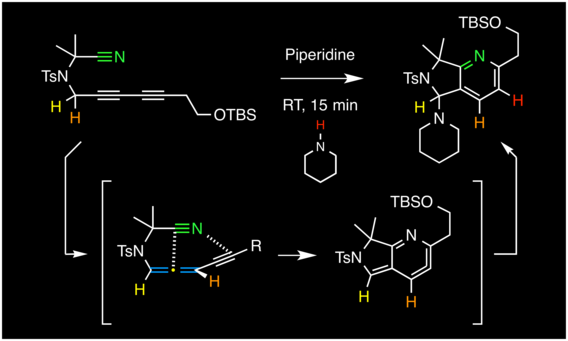
Wang, T.; Naredla, R. R.; Thompson, S. K.; Hoye, T. R. Nature 2016, 532, 484–488.
Reactions of HDDA-derived benzynes with sulfides: Mechanism, modes, and three-component reactions
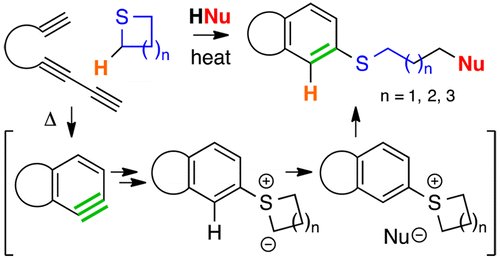
Chen, J.; Palani, V.; Hoye, T. R. J. Am. Chem. Soc. 2016, 138, 4318–4321.
Isolation and characterization of sclerienone C from Scleria striatinux
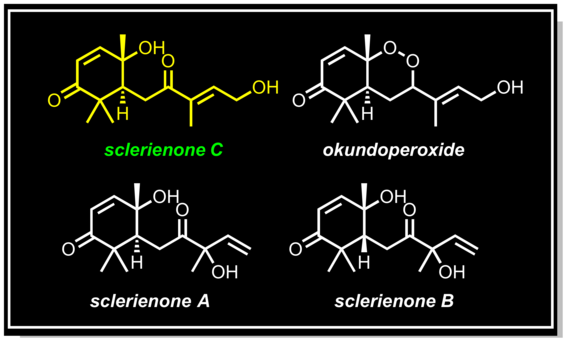
Nyongbela, K. D.; Makolo, F. L.; Hoye, T. R.; Efange, S. M. Nat. Prod. Commun. 2016, 11, 5–6.
Nanoparticles containing high loads of paclitaxel silicate prodrugs: Formulation, drug release, and anti-cancer efficacy
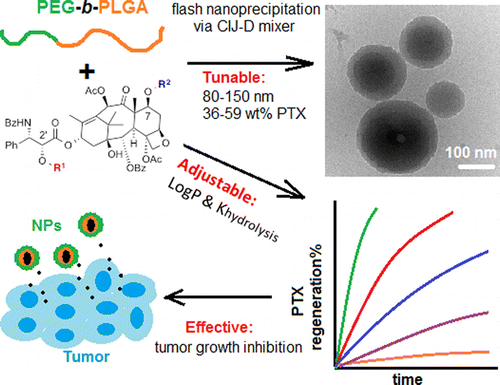
Han, J.; Michel, A. R.; Lee, H. S.; Kalscheuer, S.; Wohl, A.; Hoye, T. R.; McCormick, A. V.; Panyam, J.; Macosko, C. W. Mol. Pharmaceutics 2015, 12, 4329–4335.
Mechanism of the intramolecular hexadehydro-Diels–Alder reaction
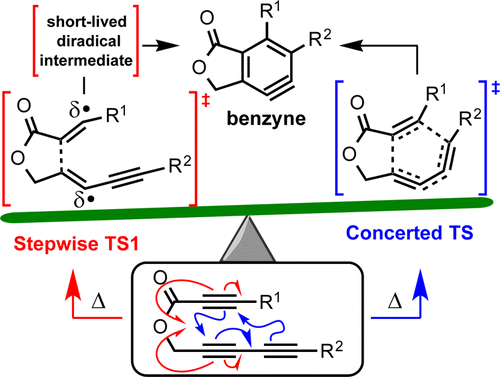
Marell, D. J.; Furan, L. R.; Woods, B. P.; Lei, X.; Bendelsmith, A. J.; Cramer, C. J.; Hoye, T. R.; Kuwata, K. T. J. Org. Chem. 2015, 80, 11744–11754.
Invited contribution to special issue: 50 Years and Counting: The Woodward-Hoffmann Rules in the 21st Century
Competition between classical and hexadehydro-Diels–Alder (HDDA) reactions of HDDA triynes with furan
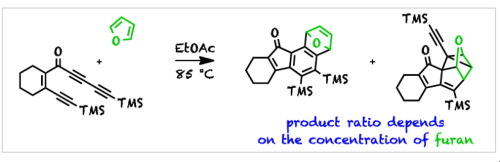
Luu Nguyen, Q.; Baire, B.; Hoye, T. R. Tetrahedron Lett. 2015, 56, 3265–3267.
We dedicate this Letter to the memory of Harry H. Wasserman, whose amalgamation of scholarship, artistry, and humanity stand as an admirable model for all to emulate.
iso-Petromyroxols: Novel dihydroxylated tetrahydrofuran enantiomers from sea lamprey (Petromyzon marinus)
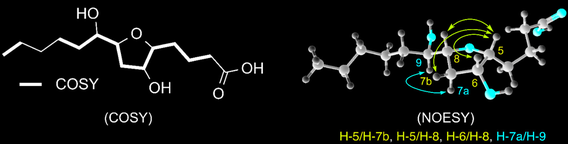
Li, K.; Brant, C.; Bussy, U.; Pinnamaneni, H.; Patel, H.; Hoye, T. R.; Li, W. Molecules 2015, 20, 5215–5222.
Differential cytotoxic activity of the petroleum ether extract and its furanosesquiterpenoid constituents from Commiphora molmol resin
Ayyad, S.E.N.; Hoye, T. R.; Alarif, W. M.; Al Ahmadi, S. M.; Basaif, S. A.; Ghandourah, M. A.; Badria, F. A. Zeitschrift für Naturforschung C. 2015, 70, 87–92.
New cytotoxic cyclic peroxide acids from Plakortis sp. marine sponge
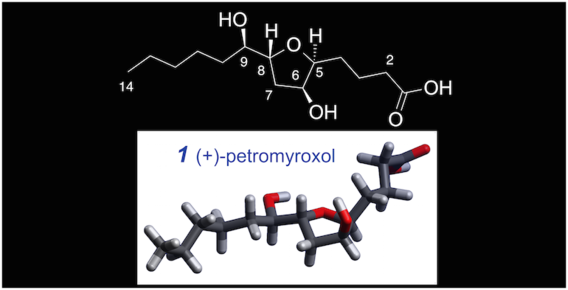
Hoye, T. R.; Alarif, W. M.; Basaif, S. S.; Abo-Elkarm, M.; Hamann, M. T.; Wahba, A. E.; Ayyad, S. N. ARKIVOC 2015, 164–175.
(+)- and (-)-Petromyroxols: Antipodal tetrahydrofurandiols from larval sea lamprey (Petromyzon marinus l.) that elicit enantioselective olfactory responses
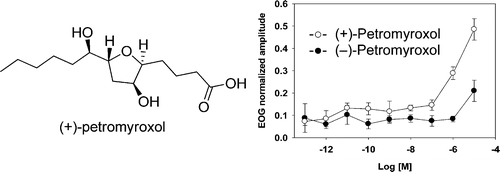
Li, K.; Huertas, M.; Brant, C.; Chung-Davidson, Y-W.; Bussy, U.; Hoye, T. R.; Li, W. Org. Lett. 2015, 17, 286–289.
Mechanism of the reactions of alcohols with o-benzynes
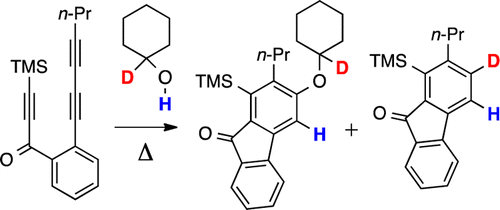
Willoughby, P. H.; Niu, D.; Wang, T.; Haj, M. K.; Cramer, C. J.; Hoye, T. R. J. Am. Chem. Soc. 2014, 136, 13657–13665.
Ultra-high-throughput screening of natural product extracts to identify proapoptotic inhibitors of Bcl-2 family proteins
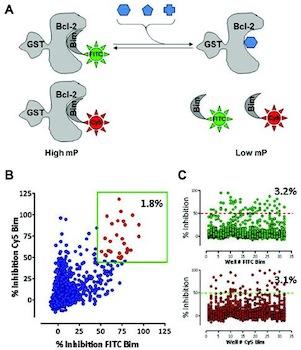
Hassig, C.A.; Zeng, F.Y.; Kung, P.; Kiankarimi, M.; Kim, S.; Diaz, P.W.; Zhai, D.; Welsh, K.; Morshedian, S.; Su, Y.; O’Keefe, B.; Newman, D.J.; Rusman, Y.; Kaur, H.; Salomon, C.E.; Brown, S.G.; Baire, B.; Michel, A. R.; Hoye, T. R.; Francis, S.; Georg, G.I.; Walters, M.A.; Divlianska, D.B.; Roth, G.P.; Wright, A.E.; Reed, J.C. J. Biomol. Screen. 2014, 19, 1201–1211.
Rates of hexadehydro-Diels–Alder (HDDA) cyclizations: Impact of the linker structure
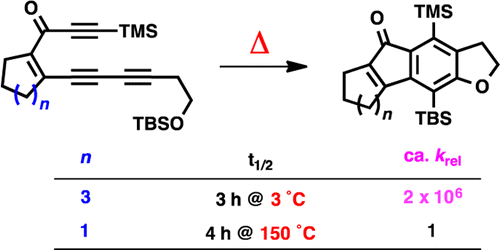
Woods, B. P.; Baire, B. ; Hoye, T. R. Org. Lett. 2014, 16, 4578–4581.
Sustainable thermoplastic elastomers from terpene-derived monomers
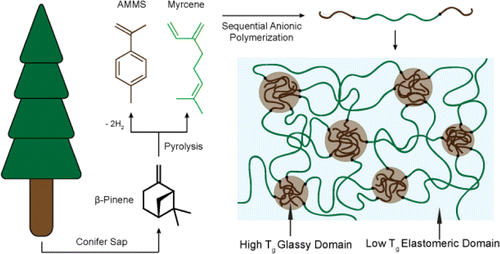
Bolton, J. M.; Hillmyer, M. A.; Hoye, T. R. ACS Macro Lett. 2014, 3, 717–720.
Silicate esters of paclitaxel and docetaxel: synthesis, hydrophobicity, hydrolytic stability, cytotoxicity, and prodrug potential
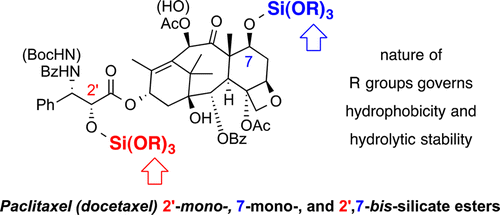
Wohl, A. R.; Michel, A. R.; Kalscheuer, S.; Macosko, C. W.; Panyam, J.; Hoye, T. R. J. Med. Chem. 2014, 57, 2368–2379.
A guide to small-molecule structure assignment through computation of (1H and 13C) NMR chemical shifts
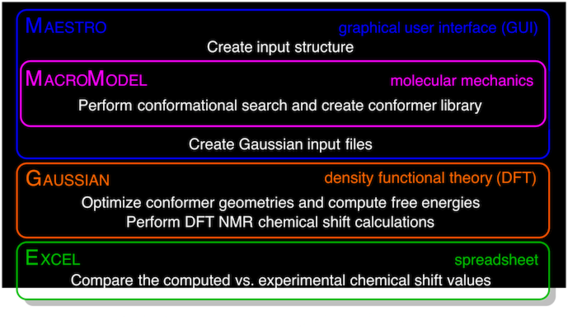
Willoughby, P. H.; Jansma, M. J.; Hoye, T. R. Nat. Protoc. 2014, 9, 643–660.
Addendum: Nat. Protoc. 2020, 15, 2277. (DOI: 10.1038/s41596-020-0293-9)
Analysis of seven-membered lactones by computational NMR methods: Proton NMR chemical shift data are more discriminating than carbon
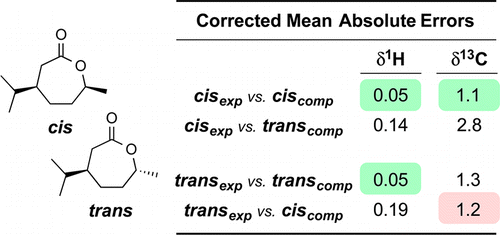
Marell, D. J.; Emond, S. J.; Kulshrestha, A.; Hoye, T. R. J. Org. Chem. 2014, 79, 753–758.
Dichlorination of (HDDA-generated) benzynes and a protocol for interrogating the kinetic order of bimolecular aryne trapping reactions
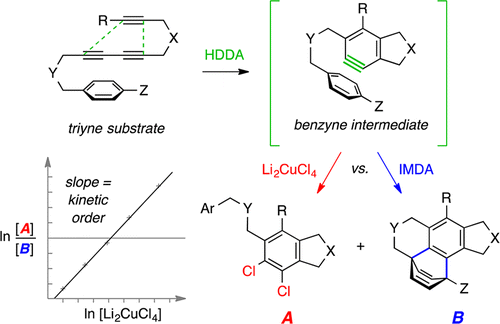
Niu, D.; Wang, T.; Woods, B. P.; Hoye, T. R. Org. Lett. 2014, 16, 254–257.
The aromatic ene reaction
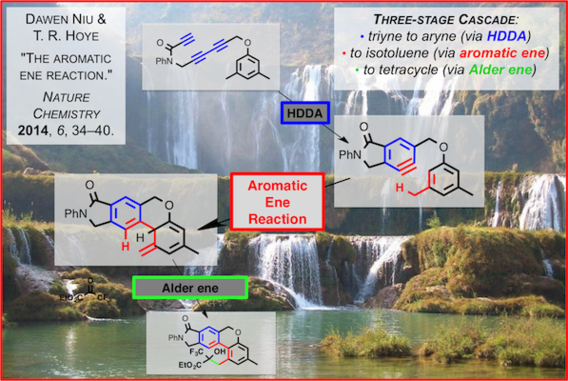
Niu, D.; Hoye, T. R. Nature Chem. 2014, 6, 34–40.
Highlighted in Synfacts 2014, 10, 258. (DOI: 10.1055/s-0033-1340776)
Flash nanoprecipitation: particle structure and stability
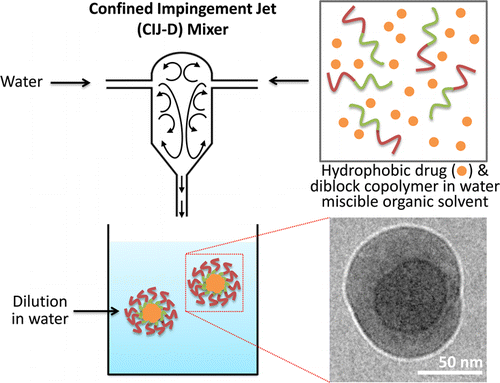
Pustulka, K. M.; Wohl, A. R.; Lee, H. S.; Michel, A. R.; Han, J.; Hoye, T. R.; McCormick, A. V.; Panyam, J.; Macosko, C. W. Mol. Pharmaceutics 2013, 10, 4367–4377.
Alkane desaturation by concerted double hydrogen atom transfer to benzyne
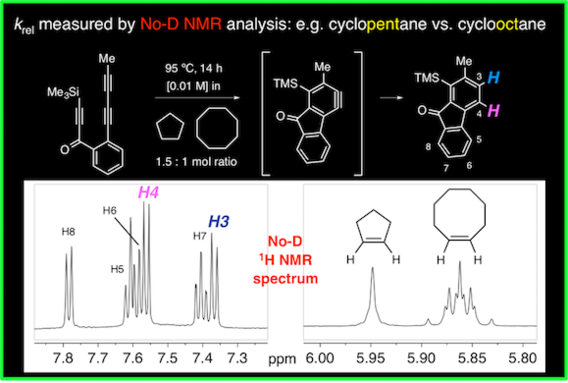
Niu, D.; Willoughby, P. H.; Baire, B.; Woods, B. P.; Hoye, T. R. Nature 2013, 501, 531–534.
Total synthesis of (±)-leuconolam: intramolecular allylic silane addition to a maleimide carbonyl group
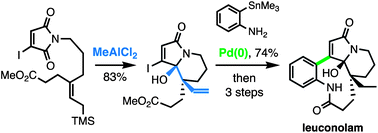
Izgu, E. C.; Hoye, T. R. Chem. Sci. 2013, 4, 2262–2266
Highlighted in Synfacts 2013, 9, 589. (DOI: 10.1005/s-0033-1338715)
New diarylheptanoids and a hydroxylated ottelione from Ottelia alismoides
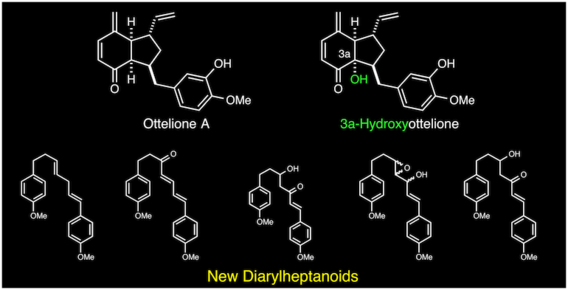
Hoye, T. R.; Ayyad, S. E. N.; Beckord, H. J.; Brown, S. G. Natural Prod. Commun. 2013, 8, 351–358.
Polycationic calixarene PTX013, a potent cytotoxic agent against tumors and drug resistant cancer
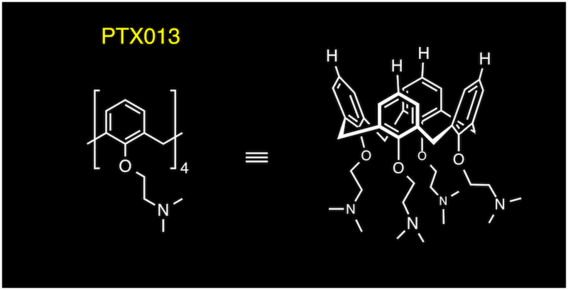
Dings, R. P. M.; Levine J. I.; Brown, S. G.; Astorgues-Xerri, L.; MacDonald, J. R.; Hoye, T. .R.; Raymond, E.; Mayo, K. H. Invest. New Drugs 2013, 31, 1142–1150.
Synthesis of complex benzenoids via the intermediate generation of o-benzynes through the hexadehydro-Diels-Alder reaction
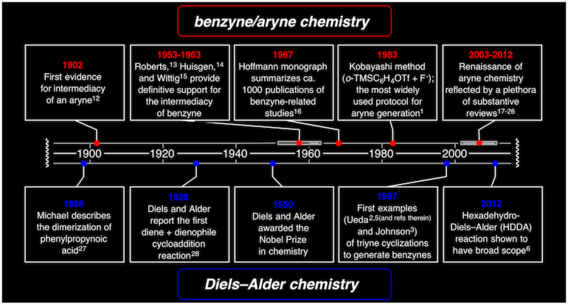
Baire, B.; Niu, D.; Willoughby, P. H.; Woods, B. P.; Hoye, T. R. Nat. Protoc. 2013, 8, 501–508.
Structure-based optimization of angiostatic agent 6DBF7, an allosteric antagonist of galectin-1
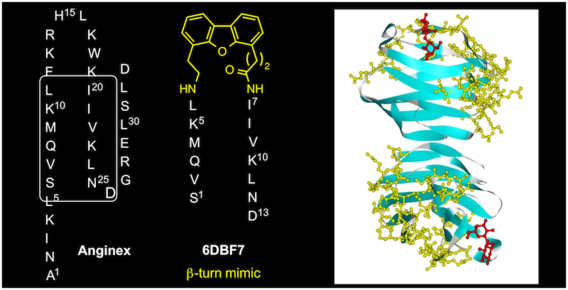
Dings, R. P. M.; Kumar, N.; Miller, M. C.; Loren, M. L.; Rangwala, H.; Hoye, T. R.; Mayo, K. H. J. Pharm. Exp. Ther. 2013, 344, 589–599.
The hexadehydro-Diels–Alder reaction
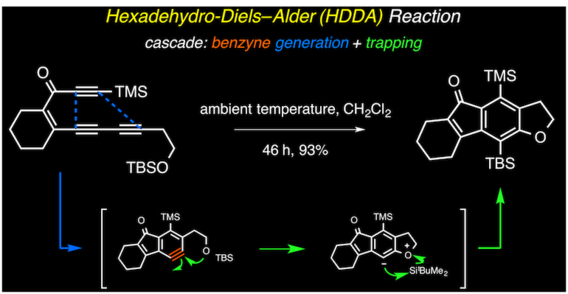
Hoye, T. R.; Baire, B.; Niu, D.; Willoughby, P. H.; Woods, B. P. Nature 2012, 490, 208–212.
Highlighted in Synfacts 2013, 9, 153. (DOI: 10.1005/s-0033-1318087)
Polyurethanes based on renewable polyols from bioderived lactones
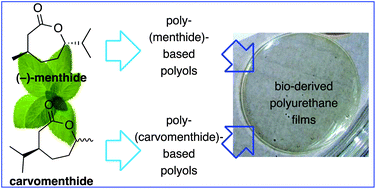
Gurusamy-Thangavelu, S. A.; Emond, S. J.; Kulshrestha, A.; Hillmyer, M. A.; Macosko, C. W.; Tolman, W. B.; Hoye, T. R. Polymer Chemistry 2012, 3, 2941–2948.
Effects of block copolymer properties on nanocarrier protection from in vivo clearance

D'Addio, S. M.; Saad, W.; Ansell, S. M.; Squiers, J. J.; Adamson, D. H.; Herrera-Alonso, M.; Wohl, A. R.; Hoye, T. R.; Macosko, C. W.; Mayer, L. D.; Vauthier, C.; Prud'homme, R. K. J. Control. Release 2012, 162, 208–217.
A simple confined impingement jets mixer for flash nanoprecipitation
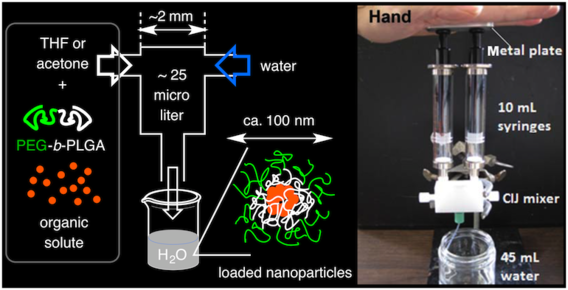
Han, J.; Zhu, Z.; Qian, H.; Wohl, A. R.; Beaman, C. J.; Hoye, T. R.; Macosko, C. W. J. Pharm. Sci. 2012, 101, 4018–4023.
Case study of empirical and computational chemical shift analyses: Reassignment of the relative configuration of phomopsichalasin to that of diaporthichalasin
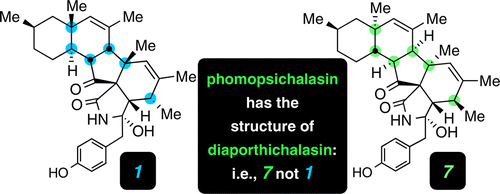
Brown, S. G.; Jansma, M. J.; Hoye, T. R. J. Nat. Prod. 2012, 75, 1326–1331.
Anti-tumor agent calixarene 0118 targets human galectin-1 as an allosteric inhibitor of carbohydrate binding
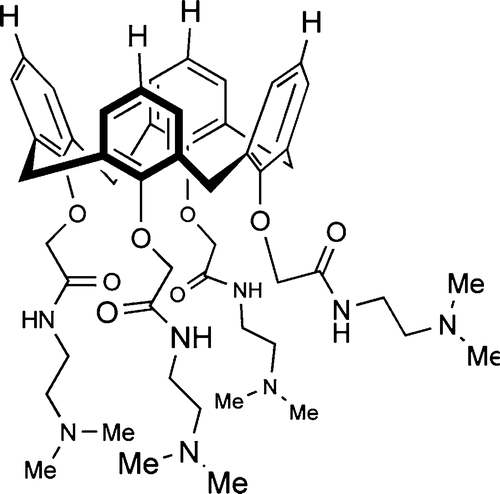
Dings, R. P. M.; Miller, M.; Nesmelova, I. V.; Astorgues-Xerri, L.; Kumar, N.; Serova, M.; Chen, X.; Raymond, E.; Hoye, T. R.; Mayo, K. H. J. Med. Chem. 2012, 55, 5121-5129.
A concise total synthesis of (±)- and (–)-okilactomycin D
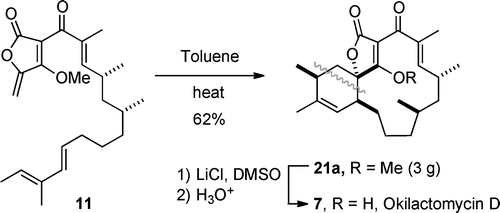
Niu, D.; Hoye, T. R. Org. Lett. 2012, 14, 828–831.
Highlighted in the following editorial: Terpenoid- and shikimate-derived natural product total synthesis: A personal analysis and commentary on the importance of the papers that appear in this virtual issue. Hale, K. J. Org. Lett. 2013, 15, 3181–3198.