Wang, T.; Hoye, T. R. J. Am. Chem. Soc. 2016, 138, 13870–13873.
Abstract
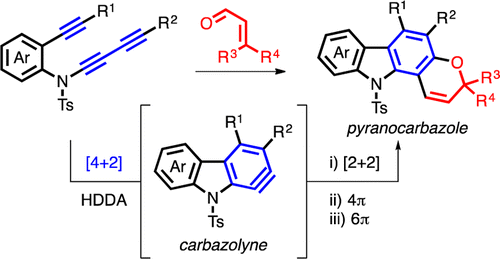
Here we report the use of the hexadehydro-Diels–Alder (HDDA) reaction for the de novo construction of a benzenoid ring in fused polycyclic heteroaromatic carbazole (i.e., [2,3]-benzoindole) skeletons. The strategy allows creation of highly substituted benzenoids. We also describe the HDDA-enabled chemical synthesis of the natural product alkaloids mahanimbine and koenidine. Trapping of the intermediate carbazolyne with a conjugated enal, proceeding through formal [2+2] cycloaddition, 4π-electrocyclic ring opening, and 6π-electrocyclic ring-closing events, constitutes a robust method for producing pyranocarbazoles.