Niu, D.; Wang, T.; Woods, B. P.; Hoye, T. R. Org. Lett. 2014, 16, 254–257.
Abstract
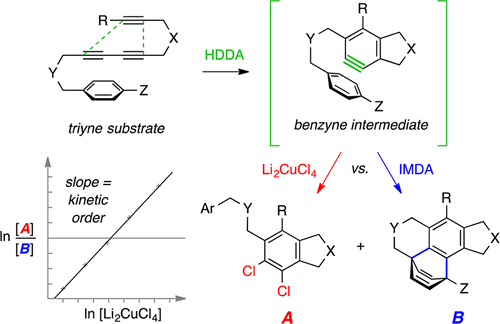
The efficient dichlorination of benzynes prepared by the hexadehydro-Diels–Alder (HDDA) reaction is reported. Cycloisomerization of a triyne substrate in the presence of dilithium tetrachlorocuprate is shown to provide dichlorinated products A by capture of the benzyne intermediate. A general strategy for discerning the kinetic order of an external aryne trapping agent is presented. It merely requires measurement of the competition between bimolecular vs unimolecular trapping events (here, dichlorination vs intramolecular Diels–Alder (IMDA) reaction to give A vs B, respectively) as a function of the concentration of the trapping agent.