Xiao, X.; Wang, T.; Xu, F.; Hoye, T. R. Angew. Chem. Int. Ed. 2018, 57, 16564–16568.
Abstract
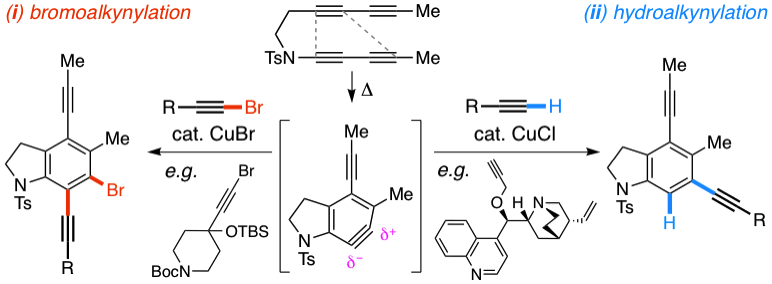
Benzynes formed by heating a suitable triyne (or tetrayne) substrate are shown to react with in situ‐generated alkynyl copper species. The latter are compatible with the polyyne substrates and two types of chemistries have been achieved (i) 1‐bromo‐1‐alkynes efficiently undergo net bromoalkynylation of the (unsymmetrical) benzynes and (ii) in situ‐generated alkynylcopper species give rise to hydroalkynylation products. The regiochemical preferences of these two modes of reaction are complementary to one another with respect to the position of alkynyl substituent in the final products.