Arora, S.; Sneddon, D. S.; Hoye, T. R. Eur. J. Org. Chem. 2020, 2379–2383.
Abstract
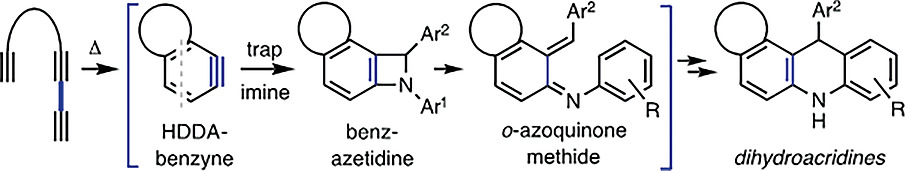
Benzynes generated by the hexadehydro‐Diels–Alder (HDDA) reaction are shown to capture C,N‐diarylimines to produce 1,4‐dihydroacridine products. The reaction is thought to proceed via benzazetidine intermediates that then undergo electrocyclic ring‐opening and ‐closing (and rearomatization) to give the dihydroacridines. These were easily oxidized with MnO2 to provide structurally complex acridines.