Zhu, C.; Hoye, T.R. J. Am. Chem. Soc. 2022, 144, 7750–7757.
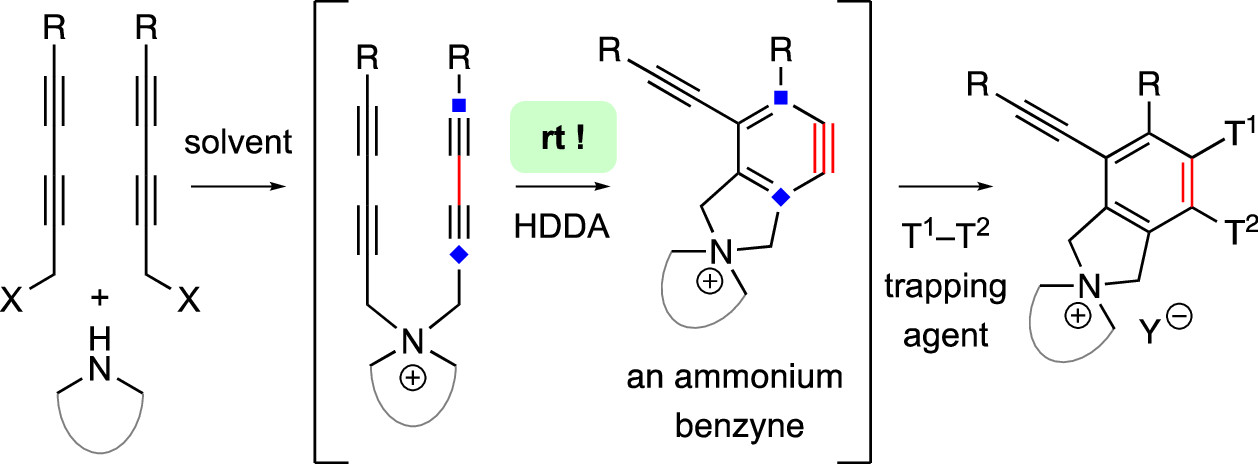
The hexadehydro-Diels–Alder (HDDA) reaction converts a 1,3-diyne bearing a tethered alkyne (the diynophile) into bicyclic benzyne intermediates upon thermal activation. With only a few exceptions, this unimolecular cycloisomerization requires, depending on the nature of the atoms connecting the diyne and diynophile, reaction temperatures of ca. 80–130 °C to achieve a convenient half-life (e.g., 1–10 h) for the reaction. In this report, we divulge a new variant of the HDDA process in which the tether contains a central, quaternized nitrogen atom. This construct significantly lowers the activation barrier for the HDDA cycloisomerization to the benzyne. Moreover, many of the ammonium ion-based, alkyne-containing substrates can be spontaneously assembled, cyclized to benzyne, and trapped in a single-vessel, ambient-temperature operation. DFT calculations provide insights into the origin of the enhanced rate of benzyne formation.