Pehere, A. D.; Xu, S.; Thompson, S. K.; Hillmyer, M. A.; Hoye, T. R. Org. Lett. 2016, 18, 2584–2587.
Abstract
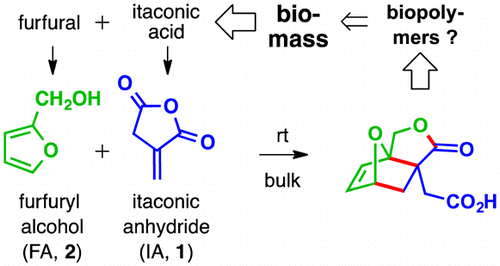
Unfavorable thermodynamics often render furans reluctant to engage in high-yielding Diels–Alder (DA) cycloaddition reactions. Here, we report the highly efficient conversion of the biosourced reactants itaconic anhydride (IA) and furfuryl alcohol (FA) to a single DA adduct. The free energy advantages provided by anhydride ring opening and crystal lattice energy of the product overcome the loss of aromaticity of the furanoid diene. Detailed 1H NMR studies provided valuable insights about relevant kinetic and thermodynamic features.