Kraemer, N.; Eason, E. M.; Hoye, T. R. J. Org. Chem. 2023, 88, 12716–12726.
Abstract
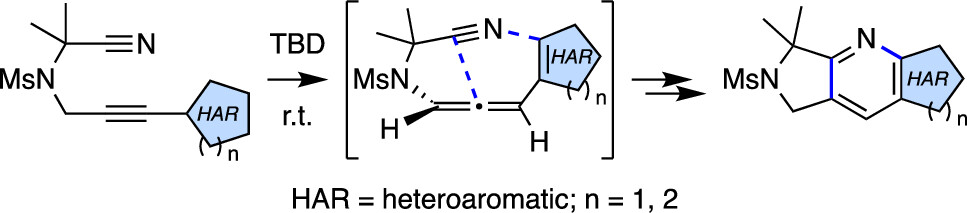
Heterocyclic substrates containing a conjugated alkyne and a pendant nitrile were shown to cyclize in an overall tetradehydro-Diels–Alder reaction to give products in which the initial heterocycle bears a newly fused pyridine ring. Base-promoted tautomerization of the alkyne to its isomeric allene allows this process to occur at ambient temperature. DFT studies support many of the mechanistic interpretations of the overall results.