Zhang, J.; Niu, D.; Brinker, V. A.; Hoye, T. R. Org. Lett. 2016, 18, 5596–5599.
Abstract
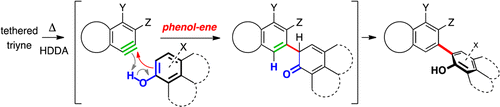
Benzynes produced thermally by the cycloisomerization of triyne-containing precursors [i.e., by the hexadehydro-Diels–Alder (HDDA) reaction] react with phenols at the carbon ortho to the hydroxyl in an enelike fashion. Following tautomerization of the intermediate cyclohexadienones, this produces biaryl derivatives. DFT calculations of model reactions support this mechanistic interpretation. Substituted, unsymmetrical phenols and bis-phenols react in a fashion that can be explained by engagement of the most readily available (non-hydrogen-bonded) hydroxyl in the phenol–ene process.