Kaicharla, T.; Lee, S.; Wang, R.; Pehere, A. D.; Xu, S.; Hoye, T. R. Arkivoc 2024, 202312081.
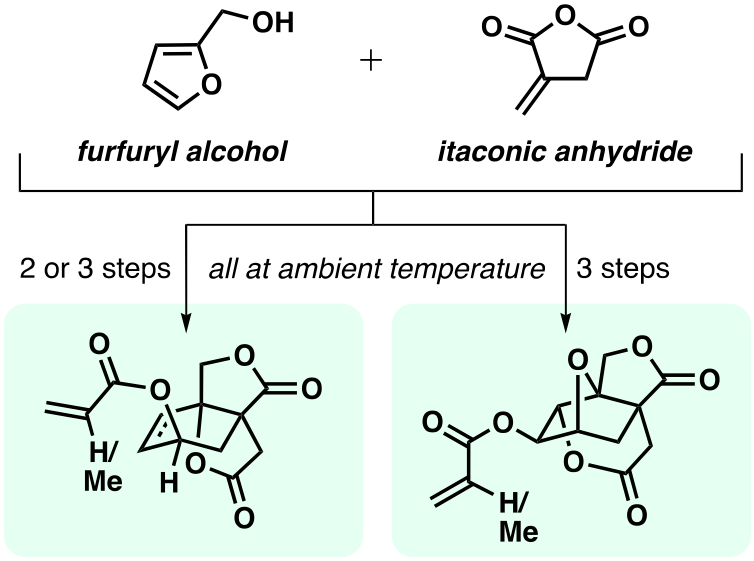
The plant-derived compounds furfuryl alcohol and itaconic anhydride are known to undergo a Diels-Alder reaction at room temperature and in bulk to efficiently give an alkene-containing lactone carboxylic acid. Reported here is the conversion of this substance to a variety of derivatives via hydrogenation, epoxidation, or halolactonization reactions. Most notable is the formation of a set of three related acrylate or methacrylate esters (see graphical abstract) produced by direct acylative ring opening of ether bonds using Sc(OTf)3 and (meth)acrylic anhydride. These esters are viewed as promising candidates for use as biorenewable monomers in reversible addition-fragmentation chain transfer (RAFT) polymerization reactions.