Pogula, V. D.; Wang, T.; Hoye, T. R. Org. Lett. 2015, 17, 856–859.
Abstract
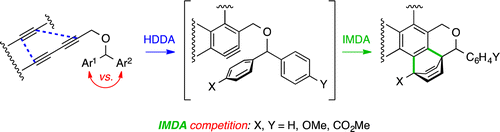
We report here the efficient, intramolecular trapping in a Diels–Alder (DA) sense of thermally generated benzynes by one of two pendant arene rings. A more electron-rich ring (p-methoxyphenyl) reacted substantially faster than a simple phenyl ring, which was, in turn, slightly more reactive vs a 4-carbomethoxyphenyl ring. Photoinduced di-π-methane rearrangement of the initial DA adducts gave rise to unusual isomeric polycyclic adducts.